Sterilization using high-pressure carbon dioxide
Year:
Abstract:
Disclosed are compositions and methods for the biocompatible sterilization of materials, in particular, of medical devices and implants. Sterilization is achieved by deactivation of microorganisms through treatment of the material with a mixture of at least one microbiocidal additive and a high-pressure or supercritical fluid, for example, high-pressure carbon dioxide or supercritical carbon dioxide. This abstract is intended as a scanning tool for purposes of searching in the particular art and is not intended to be limiting of the present invention.
Type of document:
Language:
(19) United States
US 20 1 00080790A1
(12) Patent Application Publication (10) Pub. No.: US 2010/0080790 A1
Matthews et al.
(43) Pub. Date: Apr. 1, 2010
(54) STERILIZATION USING HIGH-PRESSURE
CARBON DIOXIDE
(75) Inventors: Michael A. Matthews,
Blythewood, SC (U S); Jian Zhang,
West Columbia, SC (U S)
Correspondence Address:
Ballard Spahr LLP
SUITE 1000, 999 PEACHTREE STREET
ATLANTA, GA 30309-3915 (US)
(73) Assignee: University of South Carolina
(21) Appl. No.: 11/995,654
(22) PCT Filed: Jul. 7, 2006
(86) PCT No.: PCT/US06/26412
§ 371 (OX1),
(2), (4) Date: Apr. 26, 2009
Ltzegg 9.}
Related U.S. Application Data
(60) Provisional application No. 60/699,007, filed on Jul.
13, 2005.
Publication Classification
(51) Int. Cl.
A61L 2/18 (2006.01)
A01N 59/04 (2006.01)
A01N 63/00 (2006.01)
A01P 1/00 (2006.01)
A61L 2/00 (2006.01)
(52) U.S. Cl. ....................... .. 424/94.61; 424/700; 422/33
(57) ABSTRACT
Disclosed are compositions and methods for the biocompat-
ible sterilization of materials, in particular, of medical devices
and implants. Sterilization is achieved by deactivation of
microorganisms through treatment of the material with a
mixture of at least one microbiocidal additive and a high-
pressure or supercritical fluid, for example, high-pressure
carbon dioxide or supercritical carbon dioxide. This abstract
is intended as a scarming tool for purposes of searching in the
particular art and is not intended to be limiting of the present
invention.
Patent Application Publication Apr. 1, 2010 US 2010/0080790 A1
?“‘.“*
$n'u'(
.-W *"‘
3 ~$§
«>1-Tr.
,....u...-.........w......m«...,
5
1.
,‘, ‘f
, ‘*3? _ ~ x V _i
’25»=T§:Qi:§2.§;. :2
US 2010/0080790 A1
STERILIZATION USING HIGH-PRESSURE
CARBON DIOXIDE
CROSS-REFERENCE TO RELATED
APPLICATIONS
[0001] This application claims the benefit of U.S. Applica-
tion No. 60/699,007, filed Jul. 13, 2005, which is hereby
incorporated herein by reference in its entirety.
ACKNOWLEDGEMENT
[0002] This invention was made with government support
under Grant R01 EB 055201 awarded by the National Insti-
tutes of Health. The government has certain rights in the
invention.
BACKGROUND
[0003] Sterilization of medical devices that are used in
intimate contact with the human body is crucial to aid in
prevention of patient infection. Sterilization of medical
devices and implants is a serious issue for surgical wards and
hospitals. For example, in the United States, over 600,000
arthroplasties are performed each year, of which 0.6-2.3%
result in infection. These infections can cause substantial
physical injury or even death to the patient. Some other
widely used medical devices, such as endoscopes, can also
cause infection if not properly sterilized. Disinfection of heat-
sensitive biomaterials, especially polymers, presents a chal-
lenge to the currently used techniques of microorganism
destruction. There is increasing interest in the use ofbiopoly-
mers, autograft tissue, and allograft tissue in, for example,
regenerative medicine, and these materials generally require
sterilization prior to implantation.
[0004] Current methods of sterilization typically employ
high temperatures, toxic chemicals, strong radiation, or
strongly oxidizing chemical additives that are detrimental to
such materials and that may corrode or damage the materials
of construction of biomedical devices and sterilization equip-
ment.
[0005] In medical practice, standard sterilization methods
include steam sterilization, gamrna-irradiation, ethylene
oxide, and hydrogen peroxide sterilization. Most of these
techniques can have serious drawbacks. For example, steam
autoclaving damages heat-sensitive materials and deposits an
oxide layer onto metallic surfaces. Gamma-irradiation
reduces shear and tensile strength, elastic modulus, and trans-
parency of medical polymers by breaking polymer chains and
the resultant reactions of the free radicals produced. Ethylene
oxide not only changes the material properties of polymers
but also requires special safety considerations because of its
flammability and toxicity. Hydrogen peroxide is recognized
as a sterilant only when used in relatively high concentration
in aqueous solution, and with relatively long contact times.
The aqueous solution itself is a strong irritant. Because of the
various drawbacks associated with current sterilization tech-
niques, the next generation of polymeric medical devices and
heat sensitive biomaterials call for the use of new sterilization
methods.
[0006] Treatment with liquid or supercritical carbon diox-
ide (CO2) in the dense state can be an effective way to destroy
certain vegetative bacteria; however, several species of
spores, such as Bacillus sublilis and Geobacillus szearozher—
mophilus, have proven to be highly resistant to sterilization
methods with high-pressure CO2 alone. Due to the high resis-
Apr. 1,2010
tance of spores to treatment with pure CO2, some combina-
tion of elevated pressure, high temperature, or extended treat-
ment time are typically required to achieve a significant
reduction in the number of surviving, active spores. However,
excessively high pressure and/or temperature can damage
heat-sensitive materials and devices, and increase investment
and operating cost. Therefore, it can be desirable to operate
the sterilization process at a relatively low temperature and
pressure, and for a relatively short period of time.
[0007] Therefore, there remains a need for methods and
compositions that overcome these deficiencies and that effec-
tively sterilize bacteria and bacterial spores.
SUMMARY
[0008] Disclosed are methods and compositions related to
sterilization of microorganisms including but not limited to
bacterial spores with compositions comprising the combina-
tion of pressurized carbon dioxide and microbiocidal addi-
tives.
[0009] Also disclosed are methods for sterilizing a material
having microorganisms to be inactivated comprising the steps
of contacting the material with a mixture comprising a non-
oxidative microbiocidal additive and high-pressure carbon
dioxide or supercritical carbon dioxide, and maintaining the
contact for a period of time effective to achieve a degree of
inactivation of the microorganisms exceeding 2 log orders.
[0010] Also disclosed are methods for sterilizing a material
having microorganisms to be inactivated comprising the steps
of contacting the material with a mixture comprising hydro-
gen peroxide and supercritical carbon dioxide, and maintain-
ing the contact for a period of time effective to achieve a
degree of inactivation of the microorganisms exceeding 2 log
orders.
[0011] Also disclosed are compositions for sterilizing a
material, comprising a non-oxidative microbiocidal additive
and high-pressure carbon dioxide or supercritical carbon
dioxide.
[0012] Also disclosed are methods for sterilizing a material
having microorganisms to be inactivated comprising the steps
of contacting the material with a mixture comprising a non-
oxidative microbiocidal additive and a high-pressure or
supercritical fluid, and maintaining the contact for a period of
time effective to achieve a degree of inactivation of the micro-
organisms exceeding 2 log orders.
[0013] Also disclosed are compositions for sterilizing a
material comprising a non-oxidative microbiocidal additive
and a high-pressure or supercritical fluid.
BRIEF DESCRIPTION OF THE DRAWINGS
[0014] The accompanying drawings, which are incorpo-
rated in and constitute a part of this specification, illustrate
several embodiments and together with the description illus-
trate the disclosed compositions and methods.
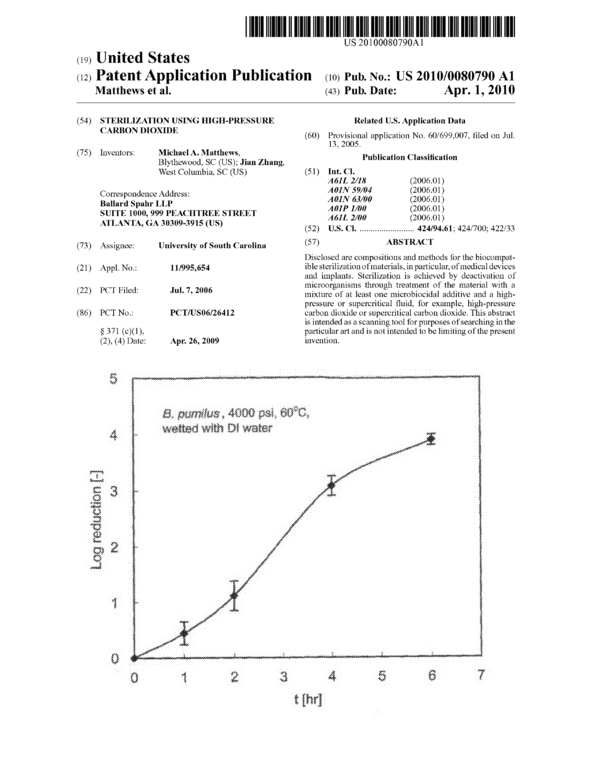
[0015] FIG. 1 shows the effect of treatment time using CO2
on the deactivation of the water-wetted spores.
[0016] FIG. 2 shows a schematic of an exemplary high-
pressure or supercritical fluid apparatus.
DETAILED DESCRIPTION
[0017] Before the present compounds, compositions,
articles, devices, and/ or methods are disclosed and described,
it is to be understood that they are not limited to specific
synthetic methods unless otherwise specified, or to particular
US 2010/0080790 A1
reagents unless otherwise specified, as such may, of course,
vary. It is also to be understood that the terminology used
herein is for the purpose of describing particular embodi-
ments only and is not intended to be limiting.
A. DEFINITIONS
[0018] As used in the specification and the appended
claims, the singular forms “a,” “an” and “the” include plural
referents unless the context clearly dictates otherwise. Thus,
for example, reference to “an additive” includes mixtures of
two or more such additives, and the like.
[0019] Ranges can be expressed herein as from “about” one
particular value, and/or to “about” another particular value.
When such a range is expressed, another embodiment
includes from the one particular value and/or to the other
particular value. Similarly, when values are expressed as
approximations, by use of the antecedent “about,” it will be
understood that the particular value forms another embodi-
ment. It will be further understood that the endpoints of each
of the ranges are significant both in relation to the other
endpoint, and independently of the other endpoint. It is also
understood that there are a number of values disclosed herein,
and that each value is also herein disclosed as “about” that
particular value in addition to the value itself. For example, if
the value “10” is disclosed, then “about 10” is also disclosed.
It is also understood that when a value is disclosed that “less
than or equal to” the value, “greater than or equal to the value”
and possible ranges between values are also disclosed, as
appropriately understood by the skilled artisan. For example,
if the value “10” is disclosed the “less than or equal to 10” as
well as “greater than or equal to 10” is also disclosed. It is also
understood that throughout the application, data are provided
in a number of different formats and that these data represent
endpoints and starting points, and ranges for any combination
of the data points. For example, if a particular data point “10”
and a particular data point “15” are disclosed, it is understood
that greater than, greater than or equal to, less than, less than
or equal to, and equal to 10 and 15 are considered disclosed as
well as between 10 and 15. It is also understood that each unit
between two particular units are also disclosed. For example,
if 10 and 15 are disclosed, then 11, 12, 13, and 14 are also
disclosed.
[0020] “Optional” or “optionally” means that the subse-
quently described event or circumstance may or may not
occur, and that the description includes instances where said
event or circumstance occurs and instances where it does not.
[0021] “High-pressure carbon dioxide,” “high-pressure
CO2,” “dense-phase carbon dioxide,” and “dense-phase CO2”
refer to pressurized, liquid carbon dioxide near but below the
critical temperature and near but below the critical pressure.
For example, in one aspect, the pressure can be from about
400 pounds per square inch (about 27.6 bar) to about 1,070
pounds per square inch (about 73 .7 bar). In another aspect, the
pressure can be from about 500 pounds per square inch (about
34.5 bar) to about 850 pounds per square inch (about 58.6
bar). In a further aspect, the pressure can be from about 600
pounds per square inch (about 41 .36 bar) to about 750 pounds
per square inch (about 51.7 bar).
[0022] “Supercritical CO2” refers to pressurized, fluid car-
bon dioxide at or above the critical temperature (about 31.1°
C.) and at or above the critical pressure (about 73.8 bar).
[0023] “High-pressure fluid” and “dense-phase fluid” refer
to any pressurized liquid near but below its critical tempera-
ture and near but below its critical pressure. In one aspect, the
Apr. 1,2010
pressure can be from 35% to 99% of the critical pressure of
the fluid, for example, from 40% to 85% of the critical pres-
sure, for example, from 60% to 75% of the critical pressure.
[0024] “Supercritical fluid” refers to a pressurized fluid at
or above its critical temperature and at or above its critical
pressure.
[0025] “Microbiocidal” refers to having the property of
inactivating pathogens or any microorganisms.
[0026] “Microbiocidal additive” or “microbiocidal agent”
as used herein, refers to having the property of inactivating
pathogens when used as an additive in a high-pressure or
supercritical fluid, in particular carbon dioxide.
[0027] “Microorganisms,” as used herein, refers to and is
understood to include active biological contaminants or
pathogens, including bacteria (including inter- and intracel-
lular bacteria, such as mycoplasmas, ureaplasmas, nanobac-
teria, chlamydia, rickettsias), yeasts, molds, fungi, spores, or
similar agents and/or single or multicellular parasites, and
combinations thereof.
[0028] Disclosed are the components to be used to prepare
the disclosed compositions as well as the compositions them-
selves to be used within the methods disclosed herein. These
and other materials are disclosed herein, and it is understood
that when combinations, subsets, interactions, groups, etc. of
these materials are disclosed that while specific reference of
each various individual and collective combinations and per-
mutation of these compounds may not be explicitly disclosed,
each is specifically contemplated and described herein. For
example, if a particular compound is disclosed and discussed
and a number of modifications that can be made to a number
of molecules including the compounds are discussed, specifi-
cally contemplated is each and every combination and per-
mutation of the compound and the modifications that are
possible unless specifically indicated to the contrary. Thus, if
a class of molecules A, B, and C are disclosed as well as a
class of molecules D, E, and F and an example of a combi-
nation molecule, A-D is disclosed, then even if each is not
individually recited each is individually and collectively con-
templated meaning combinations, A-E, A-F, B-D, B-E, B-F,
C-D, C-E, and C-F are considered disclosed. Likewise, any
subset or combination of these is also disclosed. Thus, for
example, the sub-group of A-E, B-F, and C-E would be con-
sidered disclosed. This concept applies to all aspects of this
application including, but not limited to, steps in methods of
making and using the disclosed compositions. Thus, if there
are a variety of additional steps that can be performed it is
understood that each of these additional steps can be per-
formed with any specific embodiment or combination of
embodiments of the disclosed methods.
[0029] It is understood that the compositions disclosed
herein have certain functions. Disclosed herein are certain
structural requirements for performing the disclosed func-
tions, and it is understood that there are a variety of structures
which can perform the same function which are related to the
disclosed structures, and that these structures will ultimately
achieve the same result.
[0030] Throughout this application, various publications
are referenced. The disclosures of these publications in their
entireties are hereby incorporated by reference into this appli-
cation in order to more fully describe the state of the art to
which this pertains. The references disclosed are also indi-
vidually and specifically incorporated by reference herein for
US 2010/0080790 Al
the material contained in them that is discussed in the sen-
tence in which the reference is relied upon.
B. STERILIZATION METHODS GENERALLY
[0031] The degree of deactivation of microorganisms by a
sterilization method can be characterized by log reduction.
Log reduction can be calculated with the following equation:
Log Reduction =
1 number of untreated microorganisms
o . . . .
number of surviving microorganisms after treatment
[0032] Typically, deactivation methods employing high
pressure CO2 or supercritical CO2 can deactivate B. pumilus
spores by up to three-log. However, at least six-log reduction
is required by the Federal Drug Administration (FDA) to
claim sterilization. Therefore, carbon dioxide and water alone
do not achieve adequate deactivation (Table 1).
TABLE 1
LOG REDUCTION OF B. PUMILUS SPORES ON WETTED
SPORE STRIPS WITH CO2.
DURATION PRESSURE TEMPERATURE
[HR] [BAR] [° C.] LOG REDUCTION
4 276 50 0.58 2 0.04
276 60 3.06 2 0.17
276 80 3.02 2 0.07
103 60 1.91 2 0.23
[0033] The effect of exposure time to CO2 on the deactiva-
tion of the water-wetted spores is shown in FIG. 1. The
nonlinear curve in FIG. 1 can be divided into three stages. In
the first stage (0-2 hours) the log reduction increases slowly
with the treatment time, while in the second stage (2-4 hours)
the deactivation proceeds more rapidly. In the last stage (>4
hours), the log reduction appears to approach a limiting value.
After six hours of treatment, only a 3.90-log reduction had
been obtained in the water-wetted spores. Such a long process
time is generally not ideal for a commercial sterilizer.
C. IMPROVED STERILIZATION METHODS
[0034] The present invention relates to the sterilization and
disinfection arts. It finds particular application in conjunction
with high-pressure fluids and supercritical fluids associated
with antimicrobial agents, such as sterilants or disinfectants,
for combined cleaning and sterilization or disinfection of
medical instruments, equipment, and supplies, and will be
described with particular reference thereto. It should be
appreciated, however, that the invention is also applicable to
the sterilization or disinfection of other items, including food
processing equipment and packaging and hospital supplies,
such as bed linen and protective clothing, and the like.
[0035] The disclosed compositions and methods can be
used, generally, for any surface having microorganisms. For
example, the disclosed compositions and methods can be
used with medical equipment including, without limitation,
surgical instruments, devices for implants, cannulas, endo-
scopes, syringes, bandages, medical packaging, and vials.
The disclosed compositions and methods can also be used
with tissue. The disclosed compositions and methods can also
Apr. 1,2010
be used with food processing equipment including, without
limitation, eating utensils, cookware, and food and beverage
containers. The disclosed compositions and methods can be
also used with personal items including, without limitation,
clothing, bedding, hospital and institutional bedding and
draperies, hospital and institutional towels, contact lenses,
grooming supplies, and jewelry.
[0036] The disclosed compositions and methods can be
used, generally, with substrates of any material. Materials
suitable for use with the disclosed compositions and methods
include, without limitation, metals such as aluminum, iron,
stainless steel, titanium, gold, silver, platinum, and mixtures
thereof; plastics such as polyesters, nylons, polyolefins, and
mixtures thereof; glass; stone; ceramics; and mixtures
thereof.
[0037] The disclosed compositions and methods can
achieve deactivation of microorganisms, including bacterial
spores, resulting in a log reduction of, for example, greater
than or equal to: l-log, 2-log, 3-log, 4-log, 5-log, 6-log, or
7-log.
[0038] l. Sterilizing Fluids
[0039] a. Carbon Dioxide
[0040] Carbon dioxide can be used in the disclosed method
and compositions as a high-pressure, dense or as a supercriti-
cal fluid. A supercritical fluid is a pure fluid or mixture of
fluids which is at a temperature and pressure at or above its
critical temperature and pressure.
[0041] Hi gh-pres sure sterilization involves temperatures
near but below the critical temperature and pres sures near but
below the critical pressure. Generally, such high-pressure
fluid can be in the liquid state, typically from 35% to 99% of
the critical pressure of the fluids. For carbon dioxide, sufli-
ciently high pressures can be below about 73 .8 bar, the critical
pressure of carbon dioxide.
[0042] Supercritical sterilization employs temperatures at
or above the critical temperature and pressures at or above the
critical pressure. The critical temperature and pressure vary
with the fluid selected. The critical temperature of a fluid is
the temperature above which the fluid can no longer be liq-
uefied, irrespective of the pressure applied. The critical pres-
sure is the pressure at which a substance may exist as a gas in
equilibrium with a liquid at the critical temperature. Thus, the
properties of a dense fluid change appreciably at or above the
critical pressure.
[0043] Carbon dioxide is a particularly advantageous fluid
because it is a non-polar solvent. This allows co-solvents to be
added having a high degree of selectivity. For carbon dioxide,
the critical pressure is 73.8 bar and the critical temperature is
3 1 . 1° C.
[0044] Using CO2 as a fluid to effect sterilization has sev-
eral potential benefits. First, CO2 is not flammable and is
non-toxic; the chief hazard in its use is asphyxiation. Unlike
ethylene oxide, CO2 requires no special handling or ventila-
tion, and leaves no toxic residues. Second, CO2 is inert in
most situations so it does not react with polymers, which
alleviates the aging problem caused by y-irradiation. Also,
CO2 has a low critical temperature (3l.l° C.). This is only
slightly above room temperature, so thermal degradation is
not a problem when a process is operated around the critical
temperature. In a supercritical state, CO2 has low viscosity
(about 3 to 7>X< *
Coments go here:
- Log in to post comments