Study of Thermal Properties of Cyanoacrylate Adhesives Modified with Unsaturated Compounds
Study of Thermal Properties of Cyanoacrylate Adhesives Modified with Unsaturated Compounds
Journal:
Year:
Abstract:
The effect of allyl derivatives of muconic, malonic, and isocyanuric acids, as well as some other unsaturated compounds on the properties of adhesives based on ethyl- and allyl-α-cyanoacrylates, is studied.
DOI:
10.1134/S1995421208040047
Type of document:
Language:
ISSN 1995-4212, Polymer Science, Series D. Glues and Sealing Materials, 2008, Vol. 1, No. 4, pp. 238–240. © Pleiades Publishing, Ltd., 2008.
Original Russian Text © D.A. Aronovich, A.M. Vetrova, A.P. Sineokov, 2008, published in Klei. Germetiki. Tekhnologii, 2008, No. 5, pp. 12–14.
Study of Thermal Properties of Cyanoacrylate Adhesives
Modified with Unsaturated Compounds
D. A. Aronovich, A. M. Vetrova, and A. P. Sineokov
Federal State Unitary Enterprise Kargin Institute of Polymers, Dzerzhinsk, Nizhegorodskaya obl., 60600 Russia
e-mail: niip@kis.ru
Received January 25, 2008
Abstract—The effect of allyl derivatives of muconic, malonic, and isocyanuric acids, as well as some other
unsaturated compounds on the properties of adhesives based on ethyl- and allyl-α-cyanoacrylates, is studied.
DOI: 10.1134/S1995421208040047
Cyanoacrylate adhesives with enhanced thermal stability are of interest for bonding in various fields of
technology. An analysis of the published data [1–5]
demonstrates that the basis for such adhesives is bifunctional cyanoacrylate monomers containing multiple
bonds in the alcohol radical, e.g., allyl-, propargyl-, and
allyloxyethyl cyanoacrylates. Such monomers attract
attention based on the fact that they are first cured by
the anionic mechanism using cyanoacrylate functionality; then, upon heating, multiple bonds of alcohol radical are revealed.
Moreover, thermal properties of adhesives can be
improved by the addition of thermally curing agents,
such as bi- and polyfunctional compounds containing,
e.g., (met)acrylate and allyl groups (dimethacrylate
glycols, diallylisophthalate, tri- and tetraallylpyromellitate, allyl and propargyl esters of dibasic acids, etc.).
As has been shown by us previously [6–8], composi-
tions containing derivatives of β-vinyl-α-cyanoacrylic
and cyanosorbic acids as modifiers of cyanoacrylate
adhesives are characterized by enhanced thermal properties.
In order to further study the heat resistance and
thermal stability of cyanoacrylate adhesives, we tested,
compounds containing unsaturated groups as
structuring agents, such as diallyl muconate
[CH2=CHCH2O(O)CCH=CH–]2 (I), crotolideneallyl
malonate CH3CH=CHCH=C[C(O)OCH2CH=CH2]2 (II),
and cinnamylidene malononitrile C6H5CH=CHCH=
C(CN)2 (III) (Tables 1 and 2). Structurization was performed in both the presence and absence of tert-butyl
peroxide, which can initiate crosslinking at elevated
temperatures.
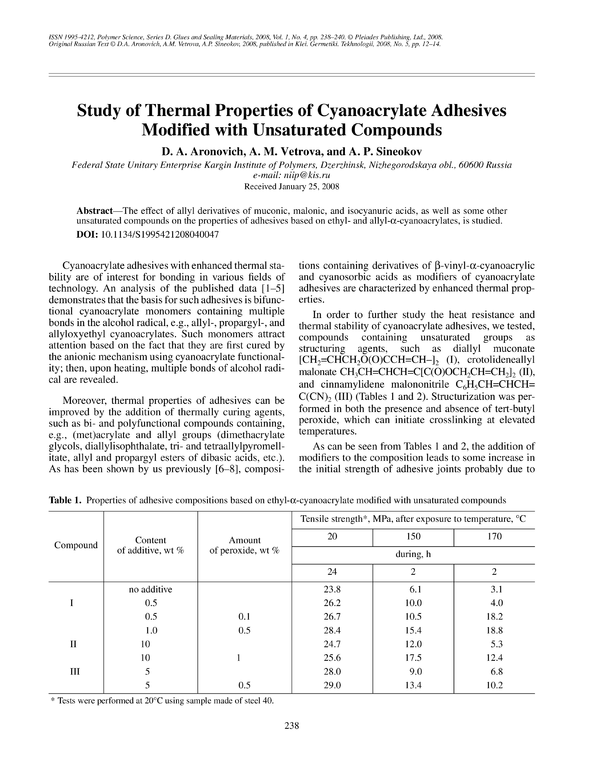
As can be seen from Tables 1 and 2, the addition of
modifiers to the composition leads to some increase in
the initial strength of adhesive joints probably due to
Table 1. Properties of adhesive compositions based on ethyl-α-cyanoacrylate modified with unsaturated compounds
Tensile strength*, MPa, after exposure to temperature, °C
Compound
Content
of additive, wt %
20
Amount
of peroxide, wt %
150
170
during, h
24
I
II
III
no additive
0.5
0.5
1.0
10
10
5
5
1
0.5
* Tests were performed at 20°C using sample made of steel 40.
238
2
23.8
26.2
26.7
28.4
24.7
25.6
28.0
29.0
0.1
0.5
2
6.1
10.0
10.5
15.4
12.0
17.5
9.0
13.4
3.1
4.0
18.2
18.8
5.3
12.4
6.8
10.2
STUDY OF THERMAL PROPERTIES OF CYANOACRYLATE ADHESIVES MODIFIED
239
Table 2. Properties of adhesive compositions based on allyl-α-cyanoacrylate modified with unsaturated compounds
Tensile strength*, MPa, after exposure to temperature, °C
ComContent
Amount
pound of additive, wt % of peroxide, wt %
20
150
170
200
during, h
24
2
6
2
6
1
6
23.0
24.0
9.2
8.4
7.7
4.0
3.2
0.5
23.5
27.4
14.8
12.6
10.0
4.8
3.7
1
0.5
25.4
20.8
12.8
13.0
9.0
9.5
5.0
10
1.0
29.6
16.6
18.8
10.5
14.0
3.0
4.1
1
30.0
28.0
11.6
5.0
30.7
26.0
20.0
16.0
9.2
30.2
28.2
22.1
17.5
10.4
no additive
I
II
5
III
5
5
0.5
3.2**
4.6
Notes: * At 20°C.
** Samples were tested at the temperature of heating.
the plasticization of polymer and the enhancement of
the thermal properties of both alkyl and allyl esters of
poly-α-cyanoacrylate. It was of interest to test unsaturated derivatives of isocyanuric acid, which can be copolymerized with allyl groups of poly-α-cyanoacrylate at
elevated temperatures, as modifiers (Table 3).
In order to synthesize isocyanuric derivatives of
β-vinyl-α-cyanoacrylic and cyanosorbic acids, we conducted the esterification of tris(hyrdoxyethyl) isocyanurate with cyanoacetic acid to form tris(cyanoacetoxyethyl) isocyanurate, which then was condensed with
acrolein or crotonic aldehyde [9]. Tris(allyloxycarboxyethyl) isocyanurate (IV) was synthesized by the
interaction between allylchloroformiate with tris(hyrdoxyethyl) isocyanurate. Prepared compounds of
tris(allyloxycarboxyethyl) (IV), tris(β-vinyl-α-cyanoacryloxyethyl) (V), and tris(cyanosorbiniloxyethyl) (VI) isocyanurates are solid substances with melting points of
63, 127, and 149°C for compounds IV, V, and VI,
respectively, and are well soluble in monomeric
cyanoacrylates. Their structures were confirmed by elemental analysis, the determination of molecular mass,
and the IR spectroscopy.
Note that the addition of compounds VII, VIII, IX,
XI, and XII, in which allyl group is directly bound to
nitrogen of triazene cycle, resulted in the spontaneous
polymerization of cyanoacrylates during storage; therefore, such compounds can be used as activators upon
their deposition onto substrates in the form of solutions
in organic solvents directly prior to bonding.
As can be seen from Table 3, compounds V and VI
increase the initial strength of adhesive joints, which is
probably due to copolymerization during bonding, as
POLYMER SCIENCE
Series D
Vol. 1
No. 4
2008
was shown in [5–7]. In addition, such compounds
increase the stability of adhesive joints to thermal
cyclic treatment at temperatures ranged from –60 to
+200°C. The best strength properties at elevated temperatures are demonstrated by the composition containing compound IV as modifier. The addition of 0.5%
tert-butyl peroxide to such composition increases the
strength of adhesive joints to 12 MPa after heating at
Tensile strength, MPa
35
30
25
1
20
15
10
2
5
3
0
10
20
30
40
50
60
Concentration of modifier, wt %
The effect of the amount of tris(allyloxycarboxyethyl)
isocyanurate (compound IV) in adhesive compositions based on allyl-α-cyanoacrylate on the strength
properties of adhesive joints after exposure for 24 h at
(1) 20, (2) 250, and (3) 300°C.
240
ARONOVICH et al.
Table 3. Strength properties of adhesive compositions based on allyl-α-cyanoacrylate containing derivatives of isocyanuric acid
Tensile strength at 20°C, MPa,
after exposure to temperature, °C
Modifier
Compound
20
R1
R2
R3
250
300
during, h
24
CH2=CHCH2OC–
–(O)OCH2CH2–
CH2=CHCH2OC–
–(O)OCH2CH2–
CH2=CHCH2OC–
–(O)OCH2CH2–
V
CH2=CHCH=C(CN)–
–COOCH2CH2–
CH3CH=CHCH=
=C(CN)–COOCH2CH2–
CH2=CH–CH2–
CH2=CH–CH2–
CH2=CH–CH2–
CH2=CHCH2=C(CN)–
–COOCH2CH2–
CH3CH=CHCH=
=C(CN)–COOCH2CH2–
CH2=CH–CH2–
CH2=CH–CH2–
CH2=CH–CH2–
CH2=CHCH2OCOCH=
=CH–COOCH2CH2–
CH2=CH–CH2–
CH2=CH–CH2–
CH2=CHCH2OCOCH=
=CH–COOCH2CH2–
CH2=CH–CH2–
C7H15OCOCH2CH2–
CH2=CHCH=C(CN)–
–COOCH2CH2–
CH3CH=CHCH=
=C(CN)–COOCH2CH2–
CH2=CH–CH2–
CNCH2CH2–
CH2=
=CHCH2OCOCH2CH2–
CH2=CHCH2OCOCH=
=CH–COOCH2CH2–
C7H15OCOCH2CH2–
C7H15OCOCH2CH2–
VI
VII
VIII
IX
X
XI
XII
3
22.7
27.1
no modifier
IV
24*
1.4
9.6
0
8.4
33.2
7.3
5.0
30.8
8.6
5.3
24.8
24.4
24.3
3.6
7.4
5.6
2.5
5.7
3.5
28.4
4.7
4.0
28.2
28.5
6.9
4.5
4.1
2.1
* Tested at 250°C.
O
R1 N
Note: 1. General formula of isocyanuric acid derivatives is
O C
C
N R2
N
C O
.
R3
2. The amount of modifier is 10 wt %
300°C, which supports the radical mechanism of
crosslinking.
We studied the effect of the content of modifier IV in
the adhesive composition based on allyl-β-cyanoacrylate
on strength properties of adhesive joints at different
temperatures (see figure). The composition containing
10 wt % modifier turned out to be stable to prolonged
thermal aging within a temperature range of 150–
250°C. Residual strength after aging at these temperatures for 500 h was equal to 9–12 MPa.
3. L. M. Pritykin, D. A. Kardashov, and V. L. Vakula,
Monomer Adhesives (Khimiya, Moscow, 1988) [in Russian].
REFERENCES
8. A. M. Vetrova, D. A. Aronovich, and A. P. Sineokov,
Klei. Germet. Tekhnol., No. 8, 22 (2007).
1. Yu. G. Gololobov and V. Gruber, Usp. Khim. 66 (11),
1054 (1997).
2. N. N. Trofimov, D. A. Aronovich, V. S. Etlis, and
N. M. Pinchuk, Plast. Massy, No. 9, 55 (1976).
4. D. L. Kotzev, T. C. Ward, and D. W. Wright, J. Appl.
Polym. Sci. 26 (6), 1941 (1981).
5. K. L. Shantha, S. Thennarasu, and N. Krishnamurti, J.
Adhes. Sci. Technol. 3 (4), 237 (1989).
6. D. A. Aronovich, Candidate’s Dissertation in Chemistry
(Dzerzhinsk, 1977).
7. D. A. Aronovich and A. M. Vetrova, Klei. Germet. Tekhnol., No. 4, 10 (2007).
9. N. M. Pinchuk, A. M. Vetrova, A. P. Sineokov, et al.,
USSR Inventor’s Certificate No. 686292A1, Byull. Izobret., No. 17 (1995).
POLYMER SCIENCE
Series D
Vol. 1
No. 4
2008
Coments go here:
- Log in to post comments