Article containing a Thixotropic Additive and Cyanoacrylate Monomer employed for developing Latent Fingerprints
Article containing a Thixotropic Additive and Cyanoacrylate Monomer employed for developing Latent Fingerprints
US4550041
Company:
Folder:
Year:
Abstract:
Latent fingerprints are developed by exposing them to vapours generated from a thin film of a storage stable cyanoacrylate monomer and a thixotropic additive in sufficient amount to render the composition substantially non-flowable. The monomer film should have a surface area at least 129 sq. cm.
An envelope package for the monomer composition has inner polyethylene surfaces. The envelope may be peeled open to expose the inner surfaces coated with a film of the composition.
Type of document:
Language:
United States Patent [19]
Thompson et al.
[54] ARTICLE CONTAINING A THIXOTROPIC
ADDITIVE AND CY ANOACRYLATE
MONOMER EMPLOYED FOR
DEVELOPING LATENT FINGERPRINTS
[75]
[73]
[21]
[22]
[60]
[51]
[52]
[53]
[56]
Inventors: Richard T. Thompson, Haddam View
Heights; Philip Hinkle, West
Hartford, both of Conn.; Robert B.
Carroll, South Paris, Me.
Assignee: Loctite Corporation, Newington,
' Conn.
App]. No.: 681,961
Fi1ed- Dec. 14, 1984
Division of Ser. No. 743,585 filed as PCT US 84/01354,
Aug. 24, 1984, published as WO 85/00963, Mar. 14,
1985, § 102(e) date Oct. 7, 1984.
Int. Cl.4 ..................... .. A61B 5/10; B41K 1/00
U.S. Cl. ................................... .. 428/35; 118/31.5;
356/71; 427/1; 428/419; 428/516
Field of Search ................. .. 427/1, 145; 118/315;
356/71; 428/35, 419, 516
References Cited
U.S. PATENT DOCUMENTS
3,380,646 4/1968 Dogen et al. ....................... .. 229/57
3,502,521 3/ 1970 Dogen et al. .. 156/69
3,523,628 1/1968 Colvin et al. .. 222/107
3,524,537 8/1970 Winter . . . . . . . . . . . . . . . . .. 206/47
3,663,501 5/1972 Adams et al. .... .. .. 260/41 R
3,839,065 10/1974 Overhults et al. .. 106/308 N
3,935,993 2/1976 Dogen et al . . . . . . . . . . . .. 229/53
3,940,362 2/ 1976 Overhults ..... .. 260/42.16
4,102,945 7/1978 Gleave .......... .. 260/879
4,180,913 1/1980 Takeuchi et al. 433/228
4,297,383 10/1981 Bourdon . . . . . . . . . . . .. 427/1
4,363,286 12/1982 Leavitt .............................. .. 427/ 1
4,379,178 4/1983 Meadows et al. ................ .. 427/ 1
4,407,842 10/1983 Shepard .................... .. 427/ 1
4,461,235 7/1984 Morton ................................. .. 427/ 1
FOREIGN PATENT DOCUMENTS
56-64933 6/1981 Japan .
OTHER PUBLICATIONS
C.A. 100(26)211222v, Oct. 1983 Takaoka Chem.
C. Abs. 100(16)122406r, Sep. 1983 Taoka Chem. Co.
lox‘
. 4,550,041
Oct. 29, 1985
Patent Number:
Date of Patent:
[11]
[45]
Chem. Abs. 100(2)8087n, Jun. 1983 Kowa K.K., JP No.
58/108135.
Chem. Abs. 99(24)196228s, Mar. 1983 Taoka Chem.
Co., JP No. 58/41068.
Chem. Abs. 96(26)218971w Taoka, Feb. 1982, JP No.
57/23666.
Chem. Abs. 96(12)80693v, Nov. 1981 Taoka, JP No.
56/142169.
Chem. Abs. 96(12)86692u, Nov. 1981 T aoka, JP No.
56/142168.
Chem. Abs. 95(18)151873z, Jun. 1981 Toagose, JP No.
56/64933.
Chem. Abs. 92(4)23838t,
Seiyaku, JP No. 54/106426.
Chem. Abs. 91(22)176339k, May 1979 Koatsu Gas, No.
54/ 57539.
Chem. Abs. 88(6)38738g, Sep. 1977 Ashiwara, JP No.
52/ 105939.
Chem. Abs. 82(8)44562g, Feb. 1974 Toagose, JP No.
74/5728.
Chem. Abs. 71(16)71543(f) 1969 Korshak et al.
Identification News: May ’82, pp. 3-5; Jun. ’82, pp. 3-4;
Jan. ’83, pp. 9-10; Feb. ’83, pp. 3-4; Feb. ’83, pp. 12, 13,
15; May ’83, p. 10.
Duraprint Investigators Report, Mar./Apr. ’83.
Police Chronicle, May ’83, “New Method of Finger-
printing . . . ”.
Payton Scientific, Undated, Visuprint Advertisment.
Police Chief, Feb. ’83, p. 9, Superprint TM ad.
Primary Examiner—I-Ierbert J. Lilling
Attorney, Agent, or Firm—Wa1ter J. Steinkraus; Eugene
F. Miller
[57] ABSTRACT
Latent fingerprints are developed by exposing them to
vapors generated from a thin film of a storage stable
cyanoacrylate monomer and a thixotropic additive in
sufficient amount to render the composition substan-
tially non-flowable. The monomer film should have a
surface area of at least 129 sq. cm.
An envelope package for the monomer composition has
inner polyethylene surfaces. The envelope may be
peeled open to expose the inner surfaces coated with a
film of the composition.
Aug. 1979 Matsumoto
8 Claims, 3 Drawing Figures
‘*7?
_\ -s
....-,
15""
;\"
/Er
U.S. Patent
Oct. 29, 1985
I20
20
‘§xu“mvL\un1“1
23
K
.\
26
4,550,041
1
ARTICLE CONTAINING A THIXOTROPIC
ADDITIVE AND CY ANOACRYLATE MONOMER
EMPLOYED FOR DEVELOPING LATENT
FINGERPRINT S
BACKGROUND OF THE INVENTION
* This is a division of Ser. No. 743,585 filed as PCT US
84/01354, Aug. 24, 1984, published as W0 85/00963,
Mar. 14, 1985, § I02(e) date Oct. 7, 1984.
To those familiar with instant adhesives, it’s no sur-
prise that their vapors can expose fingerprints with the
white residue caused by monomer “blooming”. In fact,
efforts to reduce this undesirable behavior date from the
early days of cyanoacrylate technology.
However, it was left until much later to discover that
this same process could be tumed to the noble task of
apprehending criminals through fingerprint identifica-
tion. In 1978 the Tokyo Metropolitan Police are re-
ported to have hosted a demonstration of cyanoacrylate
fuming for development of fmgerprints by criminolo-
gists of the Japanese National Police Agency. In May of
1979, Detective Inspector N. Edmunds and L. Wood of
N orthamptonshire (England) Police are reported to
have observed that fingerprints were developed when
they repaired a black plastic developing tank with Loc-
tite Super Glue TM . Within a month, they reported
their findings to a regional police conference.
Constable Paul Bourdon of the North Bay Ontario:
Police Force was an early practitioner of the method
and invented a fuming system which generates vapor in
one chamber and pumps it into another which contains
the evidence under investigation. His system has been
patented in the U.S. and Canada (U.S. Pat. No.
4,297,383, incorporated herein by reference).
Frank Kendall of the U.S. Bureau of Alcohol, To-
bacco and Firearms, developed an improvement in the
method which uses cotton treated sodium hydroxide to
accelerate the generation of vapors. A description of
this technique is given in Identification News, June
1982, page 3.
The use of heat to accelerate generation of cyanoac-
rylate vapors for fingerprint development use has also
been reported. Identification News, January 1983, page
9, and May 1983, page 10.
The cyanoacrylate vapor technique has become an
accepted method for fingerprint development. In sev-
eral cases, identifications have been made on evidence
for which no previous methods had been workable.
Recently, evidence provided by this method has been
accepted in a Kansas court leading to criminal convic-
tion. Abele, Identification News, February 1983, page
12.
Recognized benefits of using cyanoacrylate mono-
mers to develop fmgerprints which have been discov-
ered to date are as follows:
(a) Development of latent prints on objects where other
methods have failed;
(b) development of prints on difficult surfaces such as
polyethylene bags or electrical tapes;
(c) print images produced are easier to handle than
powder-dusted prints which may blow away;
(d) large enclosed areas, such as automobile interiors
can be fumed for prints.
The significant benefits of the cyanoacrylate finger-
print development technique, however, have been ac-
companied by other notable disadvantages. These dis-
advantages include the instant bonding of cyanoacry-
late adhesives to skin and clothing when contacted by
l0
15
20
25
30
35
40
45
50
55
60
65
2
evidence technicians. Also, the typical low viscosity
cyanoacrylate adhesive used in the prior techniques is
easily spilled or dripped. This not only contributes to
inadvertent bonding of clothes and skin but can also
result in damage to the evidence. Initially, without ac-
celeration of vapor generation, it has been reported that
full development of a print takes at least about five
hours and can take as long as 90 days.
With acceleration of vapor generation, other disad-
vantages have been identified as follows:
Disadvantages of Sodium Hydroxide Acceleration:
1. Sodium hydroxide is a poison and corrosive, and can
cause damage to skin if not mixed wearing gloves.
2. Preparation time is substantial.
3. The technique provides a small amount of fumes for
the amount of adhesive used. Most of the adhesive
polymerizes, within the saturated pad or as visible
white smoke, rather than being evaporated into the
atmosphere within the chamber. The polymerized
white smoke does not react with the latent print.
. Fumes from the pads rise to the top of the chamber,
then filter down, failing to provide uniform exposure
of the objects being processed to the vapors.
Disadvantages of Heat Acceleration:
1. Temperature setting can be critical. Too hot may
result in overdevelopment of the print and failure of
the adhesive to polymerize within the print. Too cool
may result in failure of the adhesive to be absorbed
into the chamber as quickly as desired, requiring
longer processing.
2. Use in smaller chambers, even in small amounts, may
result in overdevelopment if the items being pro-
cessed are not closely monitored.
3. Various methods of using heat as an accelerant also
heat the entire chamber, affecting vinyls and plastics
(especially black vinyl tapes).
4. Minor accidents involving the use of heated objects
may result in bums or damage to the chamber.
SUMMARY OF THE INVENTION
The present invention is an improvement of develop-
ing latent fmgerprints with cyanoacrylate vapors. The
method comprises subjecting an article suspected of
containing a latent fingerprint to cyanoacrylate vapors
generated from a thin film of a storage-stable composi-
tion comprising a cyanoacrylate monomer and a thixo-
tropic additive in an amount effective to render the
composition substantially nonflowable on a vertical
surface. This thixotropic composition is preferably also
stabilized against polymerization to a degree where the
adhesiveness of the composition is substantially re-
duced.
In a preferred embodiment of the invention, the gel
composition is contained in a thin envelope or pouch
which is peeled open to expose two inner surfaces
coated with a thin layer of the gel. The resulting large
surface area generates vapor rapidly and evenly pro-
longed periods of time. This rapid and sustained vapor
generation can result in development of latent fmger-
prints which are not developed by prior art methods.
The following advantages have been identified for
the inventive process over the prior art methods of
generating vapors.
1. No poisons or corrosives.
2. No preparation time.
4,550,041
3
3. Produces an even amount of exposure to the adhesive
evaporated within the chamber over a 20-minute plus
time period.
4. Provides evaporation from a larger surface area than
previous methods.
5. Room temperature operation. No critical effects of
minor variations in the temperature.
No heat to destroy vinyls or plastics.
. Overdevelopment does not occur in a short period of
time. Items can be left in the chamber up to one hour
over the amount of time required without loss of
ridge detail (items, as in previous methods, should not
be placed closer than 3 inches to the envelope).
8. No burns or damaged chambers.
9. No spillage of adhesive or contamination of evidence.
10. Substantially reduces complexity of cyanoacrylate
use making it feasible for use in the field by minimally
trained technicians.
DESCRIPTION OF THE DRAWINGS
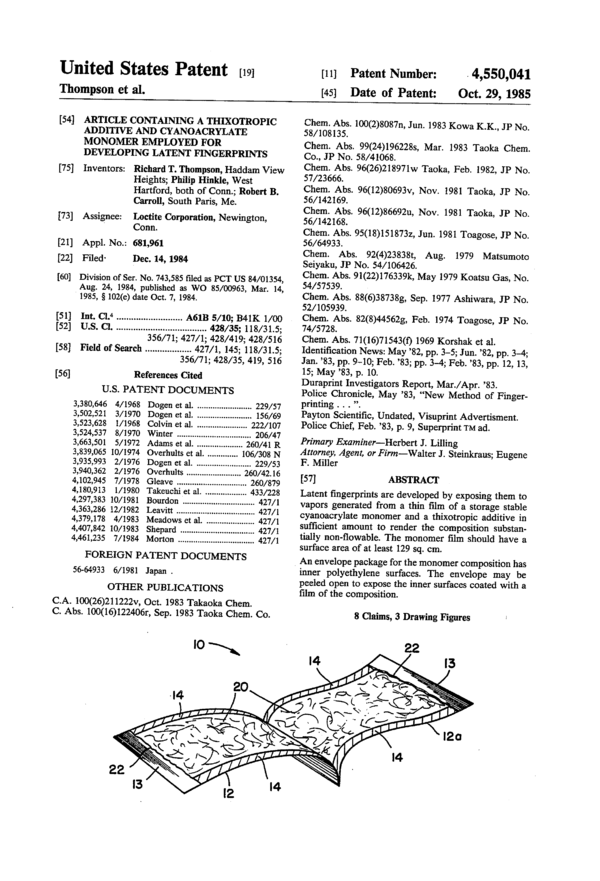
FIG. It is a side sectional view of a cyanoacrylate gel
containing package preferred for use in the inventive
method.
FIG. 2 is a bottom plan view of the package of FIG.
\-.°~
1.
FIG. 3 is a pictorial view of the opened package
showing cyanoacrylate coated inner surfaces exposed
to the air for vapor generation.
DETAILED DESCRIPTION OF THE
INVENTION
Cyanoacrylate Gel Formulation
A high vapor pressure cyanoacrylate monomer must
be utilized in order to give adequate monomer vaporiza-
tion. Methyl cyanoacrylate and ethyl cyanoacrylate are
the preferred monomers. Allyl cyanoacrylate and iso-
propyl cyanoacrylate are suitable monomers which
may also be utilized alone or in a mixture.
The formulation of a storage-stable nonflowable cya-
noacrylate gel has long been a difficult problem because
of the ease of polymerization of the monomer. Thus,
while many formulations employing typical silica thixo-
tropes have been reported in the art, such compositions
typically are intended to be utilized immediately or
have been subsequently shown to have poor shelf stabil-
ity. While it has been discovered that storage stable
formulations of certain silicas highly treated with di-
methyldichlorosilane can be made, the high degree of
surface treatment so dramatically affects the thixotropic
capability of the silica that nonflowable gels cannot be
prepared. More recently, it has been discovered that
certain uniquely treated silicas produce storage-stable
nonflowable gel compositions. Compositions of cyano-
acrylate monomers with these silicas, are described
more fully in co-pending application U.S. Ser. No.
528,275, filed Aug. 31, 1983, now U.S. Pat. No.
4,477,607, the disclosure of which is incorporated
herein by reference. The compositions of U.S. Ser. No.
528,275 are suitable for use in the inventive method and
provided the initial embodiments of the gel used in the
inventive method.
Working with the initial gel formulations, it was dis-
covered that the stabilizer level could be substantially
increased over that typically used in cyanoacrylate
adhesive formulations. Since the fingerprints are devel-
oped by polymerization of monomer vapors, the level
of stabilizer in the base composition was not critical.
Therefore, the stabilizer level in the base composition
5
10
15
20
25
30
35
40
45
50
55
60
65
4}-
could be increased to the point where adhesive bonding
is significantly reduced without affecting fingerprint
development performance. This is especially advanta-
geous in fingerprint development since the possibilities
of monomer contact are substantial. The monomer con-
tainers are open and have substantial surface areas (typi-
cally 2”>< 103)
Thixotropic Ratio 2.5 RPM/
20 RPM
92 92 92 60 94 88
88
%
I
I
8|‘5||
O\
a
~°o I
O\O'\O\
lolalll
801
3.0 3.0 5.7 5.3 7.3 5.7
'Aerosil is L! trademark of DeGussa Corp. R972, R976, R805, and 200 are fumed
silicas with respective treatments: dimethyldichlorosilane, octyltrimethoxysilane.
and untreated.
lCabosil is a trademark of Cabot Corp. N70-TS is fumed silica treated with polydi-
methylsiloxane.
-‘see US. Pat. No. 4.105.775.
The various formulations have different monomer
evaporation characteristics depending on thixotrope
and level. Formulations such as C and E are preferred
4,550,041
5
for evaporation charactertistics because they give faster
evaporation rates and almost total evaporation at room
temperature before polymerization from thin films on
polyethylene. Composition D had the slowest evapora-
tion and lowest total evaporation, presumably because
of the high polymer content.
Generating The Vapors
In order to obtain rapid and uniform vapor genera-
tion at room temperature, it is desirable that the mono-
mer be distributed over a large surface area. Whereas
development techniques using pourable liquid mono-
mers must contain the monomer in a bowl on a horizon-
tal surface, the nonflowable formulations used in the
inventive method do not have to be contained. They
may be thinly spread on vertical surfaces, e.g., on a
sheet of paper or plastic film hung on a wall, without
fear of damage to evidence or personnel.
A preferred package for the fingerprint developing
gels is shown in FIGS. 1-3. The package generally
designated by the numeral 10 is an envelope con-
structed from three sheets, 12, 12a and 13 of a laminate
material having a polyethylene inner surface. The lami-
nate is preferably a paper/polyethylene/aluminum/-
polyethylene laminate material. A bonding primer be-
tween the various layers is preferably included so that
the material does not delaminate when the envelope is
peeled open. Three heat sealed edges 14 bond sheets 12
and 12a together. Sheet 13 is heat sealed between the
end flaps 16 and 160 at the fourth end of sheets 12 and
12a, thereby forming a closed envelope structure with
separable but sealed end flaps 16 and 160. Inside the
envelope is a thin layer of the gel cyanoacrylate formu-
lation 20. To open and use, flap 16 and 16a are pulled
apart as shown in FIG. 1 and sheet 13 is tom along the
fold line 22. To facilitate substrate failure along the side
edges 14, the central outer portions of sheets 12 and 12a
may be reinforced by an additional layer of tape or label
material 23. Sheets 12 and 12a are then peeled back as
shown in FIG. 3 to open the package 10 and expose the
cyanoacrylate gel 20 to the air. The gel sticks to the
’ inner surfaces of both sheets 12 and 12a so that a maxi-
mum surface area of cyanoacrylate monomer is ex-
posed. It is recommended that the package when
opened provide a monomer coated surface area of at
least 20 sq. in. (129 sq. cm.), preferably 40 sq. in. (258 sq.
cm.) or more.
Optionally package 10 may also be supplied with a
strip 24 pressure sensitive adhesive located on the outer
surface of flap 16a. Strip 24 is covered with a release
paper 26 which may be pulled off when the package is
to be used. The exposed adhesive layer 24 may then be
used to adhere the open package to a desired surface
such as a wall or window. Since the cyanoacrylate
monomer is in gelled form it does not run off sheets 12
and 12a even when the open package is hung vertically
or upside down.
Although the seal is broken when the package is first
opened, the package may be closed after use and reused
over several weeks before the monomer is completely
polymerized or vaporized.
Developing The Fingerprints
For small portable evidence items a fuming chamber
such as an aquarium fitted with a lid may be used. The
lid contains a hanging device such as a clothes hanger
or alligator clips from which the evidence is suspended.
A preferred envelope 10 contains about 3-4 grams of a
l0
15
20
25
30
35
45
50
55
60
65
6
gel such as in formulation F in a 6£;”>
Coments go here:
- Log in to post comments