Cyanoacrylate-based Liquid Microbial Sealant Drape
Folder:
Year:
Abstract:
The invention relates to methods of using compositions for forming microbial sealant drapes. In particular, the invention relates to the use of compositions of combinations of cyanoacrylates for the in situ formation of drapes that can be used in surgery to protect patients from surgical site infections.
Type of document:
Language:
US 20100l12036A1
(19) United States
(12) Patent Application Publication (10) Pub. No.: US 2010/0112036 A1
Zhang et al. (43) Pub. Date: May 6, 2010
(54) CYANOACRYLATE-BASED LIQUID Related U.S. Application Data
MICROBIAL SEALANT DRAPE
(60) Provisional application No. 61/197,954, filed on Oct.
(75) Inventors: Sheng Zhang, Lenoir, NC (US); 31’ 2008'
Rafael R1111, H11dS011: NC (US) Publication Classification
Correspondence Address: (51) Int‘ Cl‘
STRADLEY RONON STEVENS & YOUNG, LLP ‘WK 9/7” (200601)
30 VALLEY STREAM PARKWAY, GREAT VAL- ‘WK 31/215 (200601)
LEY CORPORATE CENTER (52) U.S. Cl. ....................................... .. 424/443; 514/526
MALVERN, PA 19355-1481 (US) (57) ABSTRACT
(73) Assignee: Adhezion Biomedical, LLC., The invention relates to methods of using compositions for
Hudson, NC (U S) forming microbial sealant drapes. In particular, the invention
relates to the use of compositions of combinations of
(21) Appl. No.: 12/378,277 cyanoacrylates for the in situ formation of drapes that can be
used in surgery to protect patients from surgical site infec-
(22) Filed: Feb. 12, 2009 ti0I1S-
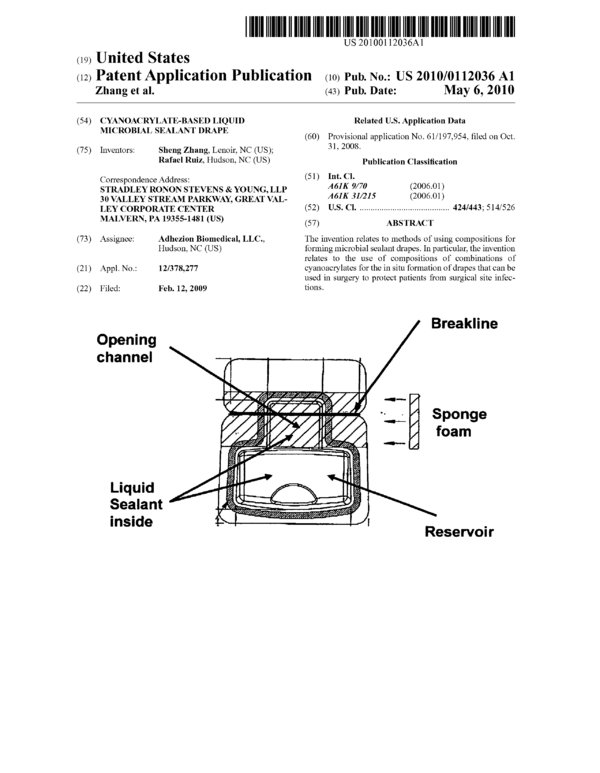
Breakl i ne
Opening
channel ‘
'“’¢i',.u-v;"v*‘**’«2'(4"/ "
./4|-.. ‘ll’; 1 ._ S
0|!’ ‘V ' ' ponge
,|J M f
. i I oam
-A _ R . --I-—-
A
Liquid
Sealant
inside
Reservoir
Patent Application Publication May 6, 2010 US 2010/0112036 A1
FIGURE 1
Breakline
Opening
channel I
-—
.,
-« .y - Sponge
. foam
Liquid
Sealant
inside
Reservoir
US 2010/0112036 A1
CYANOACRYLATE-BASED LIQUID
MICROBIAL SEALANT DRAPE
CROSS REFERENCE TO RELATED
APPLICATIONS
[0001] This application claims priority to the filing date of
U.S. Provisional Application No. 61/197954 filed Oct. 31,
2008; the disclosure of which is herein incorporated by ref-
erence.
BACKGROUND OF THE INVENTION
[0002] 1. Field of the invention
[0003] The invention relates to methods of using composi-
tions for forming microbial sealant drapes. In particular, the
invention relates to the use of compositions of combinations
of cyanoacrylates for the in situ formation of microbial seal-
ant drapes that can be used in surgery to protect patients from
surgical site infections.
[0004] 2. Description of the PriorArt
[0005] Surgical site infections (SSIs) can be classified into
two categories: (1) incisional and (2) organ, which includes
organs and spaces manipulated during an operation. Inci-
sional infections are further divided into superficial infections
and deep soft tissue-muscle and fascia infections. The Cen-
ters for Disease Control and Prevention estimates that
approximately 500,000 surgical site infections occur among
an estimated 27 million surgical procedures conducted every
year in the United States. Surgical site infections (SSI) are
listed as the second most common cause of nosocomial infec-
tion after urinary tract infections, which accounts for 40% of
hospital-acquired infections among surgical patients. Twenty
five to thirty-eight percent of all nosocomial infections
among surgical patients are estimated to be incisional surgical
site infections. SSI is a significant cause of surgical morbidity
and mortality, occurring in 2-5% of patients having clean
extra-abdominal operations and up to 20% of patients under-
going intra-abdominal procedures. Patients with SSI are
twice as likely to die, 60% more likely to be admitted to an
Intensive Care Unit, and more than 5 times more likely to be
readmitted to the hospital than patients who are not infected.
Surgical site infections result in longer hospitalization and
have large economic impact on patients and the health care
system. Patients with surgical site infections are hospitalized
an additional 7 days on average. The longer hospital stay cost
an additional $3,152 on average. The average total cost for
medical care during the eight weeks after hospital discharge is
$5,155 for patients with surgical site infections compared
with $1,773 for patients without SSIs. The total cost includes
out patient visits, pharmacy, radiology services, re-hospital-
ization, skill-nursing facility, home health aids, and durable
equipment.
[0006] The frequency of surgical site infections in patients
varies from surgeon to surgeon, hospital to hospital, surgical
procedure to surgical procedure and patient to patient. Surgi-
cal site infections can be caused by extemal sources of con-
tamination including surgical personnel, surgical environ-
ment, and surgical instruments. Most SSIs are, however,
caused by patient’s own normal skin flora which can enter the
body through the surgical incision. The patient’s skin flora is
considered as the first and foremost pathogenic source
because the transmission of bacteria from skin to the incision
is very eflicient. Innocuous bacterial flora on the skin may
also be colonized by pathogenic organisms. The bacteria of
May 6, 2010
normal skin flora can cause wound infection in the presence
of foreign materials that greatly enhance the pathogenic
potential of these bacteria. Therefore, bacterial contamina-
tion occurs predominantly during and following surgical pro-
cedures.
[0007] Different methods of preventing SSIs have been
developed to reduce patients’ surgical site infections.
Advanced surgical techniques and skillful surgeons can
reduce the duration of surgery. Operation personnel and
operation room hygiene management can lower the probabil-
ity of exogenous pathogens. Operations that are conducted
when patients have healthy physical and psychological states
may enhance patients’ immune system so that the chance of
surgical infections may be considerably reduced. Thoughtful
plans and careful selection of effective antibiotics can also
help reduce the chance of contamination of bacteria.
[0008] Topical bactericidally active or antimicrobial agents
such as iodophors, chlorhexidine, and alcohol-containing
products have been applied to the surgical site before surgery
to kill bacteria. These agents are preoperative skin prepara-
tion products, washes, surgical scrub tissues, wound cleaners,
lotions and ointments.As early as 1960s, the successful use of
prophylactic antibiotics was reported in a randomized, pro-
spective, placebo-controlled clinical study of abdominal
operations on the gastrointestinal tract. The success of anti-
biotic prophylaxis was due to the appropriate patient selec-
tion and wise choice of available agents.
[0009] U.S. Pat. No. 4,542,012 teaches the application of
antimicrobial agents by depositing antimicrobial composi-
tions onto human skin to form an antiseptic film. The antimi-
crobial composition is applied to the skin as a liquid solution
in a fugitive solvent. After the solvent evaporates, a thin film
containing antimicrobial agent is formed on the skin.
[0010] U.S. Pat. No. 5,916,882 discloses a providone-io-
dine alcohol gel antimicrobial skin-preparation formulation
which is used to disinfect a surgical site. The pre-operative
skin-preparation formulation quickly kills bacteria when
applied to the surgical site. The skin-preparation formulation
continues to effectively inhibit microorganism growth in the
applied area for a relatively long period of time. Application
of the skin-preparation formulation is controllable because
the formulation does not run when applied to a patient. The
antimicrobial skin-preparation formula includes iodine, alco-
hol and gel.
[0011] U.S. Pat. No. 6,228,354 provides a skin-preparation
composition which does not harm the skin yet promotes asep-
sis on the skin. The skin-preparation composition disclosed
has a rapid antimicrobial activity when in a liquid form and a
sustained antimicrobial activity when dry. The skin prepara-
tion composition forms a water-resistant film on skin and is
not readily removed when a wound or surgical site is sponged
or irrigated. The antimicrobial film can be removed by rub-
bing an aqueous solution having the proper pH onto the skin.
This patent describes a film-forming topical antimicrobial
composition that includes a broad spectrum antimicrobial
agent, a water-resistant polymer system, a neutralizer, a pH
sensitive polymer, and an alcohol.
[0012] U.S. Pat. No. 6,488,665 discloses an antimicrobial
skin-preparation delivery system used to disinfect a surgical
site. The antimicrobial skin-preparation formula consists of
iodine, alcohol and gel. The delivery system is composed of
an antimicrobial alcohol gel formulation contained within a
sealed, flexible container and a gel formulation dispenser
connected to the container. A porous applicator pad with
US 2010/0112036 A1
enlarged holes for passage of the gel formulation is described.
The flow rate of the gel formulation is controlled by the
external pressure applied to the flexible container.
[0013] US Patent Publication Nos. 20040126355 and
20080102053 disclose antimicrobial skin compositions com-
prised of an antimicrobial agent, water, an alcohol, and one or
more pH sensitive viscosity builders. The composition’s vis-
cosity is from 100 cp to 1,000 cp and the formulation com-
bines the advantages of an antimicrobial agent and an alcohol.
The viscosity of the formulation permits dispensing from the
applicator, while preventing the solution from flowing away
from the wound area. pH sensitive methacrylic polymers are
used as viscosity modifiers. The preparation forms a water-
resistant film that is diflicult to remove during wound irriga-
tion, but can be easily removed upon completion of the pro-
cedure.
[0014] One of the disadvantages associated with topical
application of skin preparation products is that the antimicro-
bial agents are only effective for a short period of time.
Bacteria that may have survived the initial application of skin
preparation products can proliferate and produce a large
pathogen population. In addition, appropriate antimicrobial
prophylaxis is determined by many factors such as proper
case selection, anti-microbial agent selection, dosing and
route of administration and duration of therapy. Inappropriate
use of antimicrobial agents not only increases the cost of
medical health care, but also exposes the patient to potential
toxicity and other risks. Moreover, many gram-positive
organisms isolated from patients with surgical site infections
are resistant to multiple antimicrobial agents. The problem of
antimicrobial resistance in gram-positive nosocomial patho-
gens has been a growing concem.
[0015] In addition to the use of antimicrobial skin prepara-
tion products, surgical incise drapes have also been used to
help reduce the migration of germs and bacteria into the
incision site. The surgical incise drape is usually a clear
polymeric film with an adhesive backing on one side which is
in turn covered with a release liner. Generally, the incise drape
is used in conjunction with towels or surgical drapes to main-
tain the surgical site as sterile and clean as possible in order to
inhibit surgical site infections. A continuous or longer lasting
antimicrobial effect may be obtained by combining the anti-
microbial agent with a surgical incise drape.
[0016] U.S. Pat. No. 3,579,628 discloses a hydrophilic
acrylic film dressing which contains a composition which
reacts with water to generate a bacteriostatic substance. The
hydrophilic acrylic films are particularly suitable for use as
occlusive dressings and for reducing bacteria.
[0017] U.S. Pat. Nos. 4,310,509 and 4,323,557 disclose
dermatologically acceptable compositions made of a pres-
sure-sensitive surgical incise drape and a broad-spectrum
antimicrobial agent which can be released from the drape
placed in contact with the skin. The active broad-spectrum
antimicrobial agent is polyvinylpyrrolidone-iodine complex
or chlorhexidine. The antimicrobial agents are applied onto
the surgical drape which is made of polymeric materials such
as polyurethane, polyvinyl ethers, polyesters, or polyethyl-
ene.
[0018] U.S. Pat. No. 4,340,043 discloses an adhesive-
coated incise drape material incorporating uniform amounts
of silver sulfadiazine as an antimicrobial agent. The incise
drape is made of polyurethane sheets with an adhesive layer.
[0019] U.S. Pat. No. 4,643,181 discloses a surgical dress-
ing or incise drape material comprising a substrate coated
May 6, 2010
with an antimicrobial containing adhesive. The substrate may
be a woven or knitted fabric, a nonwoven fabric, a plastic or a
polymeric film. The preferred substrate in the invention is a
polyurethane film. The antimicrobial is polyhexarnethylene
biguanide hydrochloride, which is distributed in the adhesive
as particles with a size in the range of 20 to 300 microns.
[0020] U.S. Pat. No. 5,069,907 discloses a synthetic poly-
meric film or fabric surgical drape having incorporated
therein a broad spectrum antimicrobial agent. The drape may
have an adhesive layer attached to one of its external surfaces.
The preferred antimicrobial agent used is 5-chloro-2-(2,4-
dichloro-phenyl)phenol. Suitable adhesives utilized include
polyacrylate adhesives.
[0021] U.S. Pat. No. 5,803,086 discloses adhesive coated
incise drapes useful in surgical procedures. The incise drapes
comprise a flexible film backed coating on one side with a
dermatologically acceptable pressure sensitive adhesive
(PSA) on the other side. The incise drape can be applied by
two people wherein one person holds the core and a second
person ur1rolls the drape by pulling on the handle protruding
from the opposite end of the drape.
[0022] U.S. Pat. Nos. 5,979,450; 5,985,395 and 6,742,522
provide surgical incise drapes comprising a flexible film hav-
ing a major portion thereof coated with an adhesive. The
incise drape has a leading edge and a trailing edge and further
includes a film handle at the leading edge. Methods described
include providing a drape, grasping the film handle of the
drape, pulling upon the liner to remove at least a portion of the
liner exposing at least a portion of the adhesive coating the
major portion of the flexible film, holding the surgical incise
drape in a position such that at least a portion of the adhesive
is contacting the patient, and then removing portions of the
liner remaining.
[0023] US Patent Publication Nos. 20020002223,
20040115274 and 20080078413 disclose adhesive composi-
tions containing acrylic polymers, tackifiers and a broad spec-
trum antimicrobial agent. The adhesive composition is an
essentially solventless composition. The antimicrobial agent
utilized is diiodomethyl-p-tolylsulfone with a preferred con-
centration of antimicrobial agents in the adhesive of about
0.1% to about 2% loading by weight. The antimicrobial adhe-
sive composition is included in a polymeric substrate to form
a surgical drape. The polymeric substrate is preferably a
polyester or co-polyester sheet material.
[0024] US Patent Publication No. 20050284487 discloses a
draping product, which is coated with adhesive along at least
one edge. The adherence strength of the adhesive is greater
than 0.5 N/25 mm when applied to skin. The damage to
stratum comeum of the skin covered by the adhesive is less
than 30% after removal. The adhesive coating is comprised of
a pressure sensitive adhesive such as silicone elastomer, a
hydrogel or a soft, tacky hot melt adhesive.
[0025] US Patent Publication No. 20070048356 describes
an antimicrobial material composition that can be applied to
material substrates. The antimicrobial composition includes a
first or primary antimicrobial agent, such as polyhexameth-
ylene biguanide (PHMB), a second antimicrobial agent, an
anti-static agent or fluoropolymer and/ or an organic acid. The
substrate may encompass both woven and nonwoven fabrics
made from either natural or synthetic fibers, rubber, plastic,
and other synthetic polymer materials. The composition
exhibits an effective microbe-killing eflicacy within a period
of about 30 minutes.
US 2010/0112036 A1
[0026] In spite of the beneficial properties of conventional
surgical drapes with respect to inhibition bacterial infection,
there are many challenges and problems associated with the
conventional surgical drapes regardless of whether they
incorporate antimicrobial agents. Under certain circum-
stances conventional surgical drapes may actually increase
the risk of surgical site infection. Conventional surgical
drapes can be lifted during surgery which results in entry of
bacteria into the surgical site. The lifting of the conventional
surgical drape is usually caused by failure of the adhesive to
remain in contact with the patient’s skin. Attempts to increase
adhesive strength may also prove disadvantageous because
more force is then required to remove the drape from skin
leading to damage of the skin near the surgical site.
[0027] Conventional surgical drapes are normally large and
difficult to apply to the patient without wrinkling the drape
film. Wrinkling of the surgical drape at the surgical site may
block visibility, making it difficult for the surgeon to see the
incision site. In addition, the surgical drape will not prevent
microorganisms from entering the incision if the drape is
wrinkled. Wrinkling is especially problematic with applica-
tion of the conventional surgical drapes to a non-flat skin
surface such as the elbow or knee.
[0028] Incorporation of antimicrobial agents into conven-
tional surgical drapes may permit the antimicrobial action of
the agents to last longer. Antimicrobial agents currently avail-
able are, however, not effective at killing and immobilizing
pathogens on the surface to which the agents are applied. The
extensive use of antimicrobial products has raised concems
about antimicrobial resistance to antibiotics. In addition,
most antimicrobial compounds are heat labile and cannot
survive radiation sterilization. This makes it difficult to pre-
pare sterile surgical drapes infused with antimicrobial agents.
[0029] Even though many different procedures have been
applied to reduce surgical site infections, the risk of such
infections still exists because of the continuing survival of
skin bacteria after these treatments. Since endogenous flora
on patient’s skin plays a key role in the development of
surgical site infections, a simple and comprehensive solution
to the problem would be to minimize endogenous bacteria at
and around the surgical site. It is known that cyanoacrylate
polymer film can act as a mechanical barrier to penetration by
bacteria while maintaining a natural healing environment.
Cyanoacrylate monomers, which polymerize on contact with
tissue surface to provide a thin and flexible polymer film, have
been used as tissue adhesives for several decades. Cyanoacry-
late adhesives also exhibit strong bond strength and very rapid
cure time.
[0030] Cyanoacrylate’ properties as adhesives may also
make them desirable candidates as microbial sealant drapes.
Cyanoacrylate microbial sealant drapes could prevent surgi-
cal site infections by overcoming the difficulties experienced
by the conventional surgical drapes. U.S. Pat. No. 7,255,874
discloses that modified cyanoacrylate monomers can be used
in various medical applications including wound closure,
treatment of burns and abrasion and as surgical drapes. U.S.
Pat. No. 5,730,994 describes methods for draping a surgical
site by the in situ formation of cyanoacrylate polymer drape
over skin surface. While the specification describes various
cyanoacrylate monomers that can be used as surgical drapes,
the preferred compositions contained only n-butyl
cyanoacrylate. Furthermore, only n-butyl cyanoacrylate
compositions were tested as surgical drapes.
May 6, 2010
[0031] There are several shortcomings associated with
using n-butyl cyanoacrylate as a surgical drape. Compared to
longer chain alkyl cyanoacrylates, n-butyl cyanoacrylate is
less flexible and cracks more easily after forming a polymer
film. Thus a plasticizer is usually needed in the n-butyl
cyanoacrylate formulation to improve flexibility. In addition,
short-chain cyanoacrylates polymerize quickly and then
degrade rapidly into formaldehyde and the corresponding
alkyl cyanoacetate, which can cause significant histotoxicity.
Polymer films of n-butyl cyanoacrylate sloughs off from skin
faster than that of long alkyl chain cyanoacrylates. Skin irri-
tation also occurs with the use of n-butyl cyanoacrylate.
[0032] Hence, development of a cyanoacrylate-based
microbial sealant drape which can immobilize the infectious
microorganisms and effectively seal out the bacteria from a
surgical site is desired. It is desirable to have a cyanoacrylate-
based microbial sealantdrape product that can provide a um-
form and flexible film. It is also desirable to develop a
cyanoacrylate microbial sealant drape with significantly less
tissue toxicity. Additionally, it is also desirable to develop an
easy to use cyanoacrylate-based microbial sealant drape that
will last a long time after the surgery to inhibit the postopera-
tive surgical site infections.
SUMMARY OF THE INVENTION
[0033] The present invention provides cyanoacrylate-
based liquid microbial sealant drape compositions compris-
ing mixtures of cyanoacrylates to inhibit the surgical site
infections. The liquid sealant film formed upon polymeriza-
tion of the cyanoacrylate mixture prevents the spread of bac-
teria by trapping and immobilizing the microorganisms on
the surgical sites. The compositions of the present invention
provide flexible microbial sealant drapes without the addition
of plasticizers and/ or antimicrobial agents.
[0034] The present invention provides a method of per-
forming surgery with a lowered risk of contamination includ-
ing the steps of applying a preoperative skin preparation to a
surgical site, applying the microbial sealant drape composi-
tion based on liquid cyanoacrylates to the surgical site, form-
ing the microbial sealant film on the surgical site, making an
incision through the microbial sealant film, and performing
surgery.
[0035] The present invention provides liquid microbial
sealant drape compositions which can effectively reduce the
amount of microorganisms in the surgical site. Effective
immobilization of microorganisms, by liquid microbial seal-
ants of the present invention, were confirmed by both in vitro
and in vivo bacteria immobilization test. In vitro immobili-
zation test on sterile pig skin confirms that the microbial
sealant compositions of the present invention are at least 95%
effective in preventing the spread of the clinically relevant
bacteria on the surgical sites under a variety of usage condi-
tions. In preferred embodiments of the microbial sealant com-
positions of the present invention the compositions are at least
99.5% effective in preventing the spread of the clinically
relevant bacteria on the surgical sites under a variety of usage
conditions. In more preferred embodiments of the microbial
sealant compositions of the present invention the composi-
tions are at least 99.9% effective in preventing the spread of
the clinically relevant bacteria on the surgical sites under a
variety of usage conditions. The microbial sealant composi-
tions of the present invention do not need to be used in
combination with an antimicrobial surgical incise drape and
may be used as a substitute for an antimicrobial surgical
US 2010/0112036 A1
incise drape. The in vivo bacteria immobilization on 60
human subjects indicates that the microbial sealant composi-
tions of the present invention can reduce microbial coloniza-
tion by at least 99.9% within 15 minutes and maintain at least
a 99.9% reduction throughout the 24 hours post treatment.
[0036] The present invention provides microbial sealant
drape compositions which are resistant to the passage of
blood-borne pathogens using viral penetration as a test sys-
tem. No viral penetration was detected for the disclosed
microbial sealant film.
[0037] The present invention provides a method of inhibit-
ing the surgical site infections during and post the surgery. It
takes days for the microbial sealant film to slough off. The
disclosed cyanoacrylate liquid drape compositions are thus
providing the post-surgical infection protection based on the
anti-microbial property of cyanoacrylates.
[0038] The present invention provides microbial sealant
drape compositions which provide a desirable degradation
profile.
[0039] The present invention provides microbial sealant
drape compositions including at least one cyanoacrylate
monomer with longer alkyl chain and at least one cyanoacry-
late monomer with shorter alkyl chain. The desired
cyanoacrylate properties can thus be fine-tuned by combining
the longer chain and shorter chain cyanoacrylates in specific
ratios. Properties such as bonding strength, setting time, vis-
cosity, degradability and biocompatibility can be altered
depending on the specific combination of cyanoacrylates.
Mixed cyanoacrylate compositions with about 60% to about
90% or more of 2-octyl cyanoacrylate are preferred for use as
microbial sealants to prevent the surgical site infections.
[0040] The present invention provides sterile microbial
sealant drape compositions which may be sterilized by the
combination of ethylene oxide exposure and E-beam irradia-
tion. Sterile microbial sealant compositions sterilized in this
manner provide at least a two year shelf life.
[0041] The present invention provides microbial sealant
drape compositions based on mixed cyanoacrylate mono-
mers, which are packaged in a single use applicator. The
applicator includes a compartment containing the microbial
sealant composition as well as a sponge applicator tip.
Cyanoacrylate-based microbial sealant composition can be
readily dispensed onto the sponge applicator tip. A uniform
sealing film can be formed by applying the cyanoacrylate-
saturated sponge tip onto the surgical sites.
[0042] The present invention provides microbial sealant
drape compositions which are compatible with currently
available skin preparation products, surgical incise drapes
and wound closure products.
[0043] The present invention provides microbial sealant
drape compositions, which form flexible and tight films on
the substrates so that little or no detectable cyanoacrylate
residue is transferred into the incision wound by the surgical
blade.
[0044] The present invention provides microbial sealant
drape compositions which generate less heat during the appli-
cation compared to commercially available drape products
comprised of 100% butyl cyanoacrylate. The average tem-
perature increase of the skin after applying microbial sealant
composition of the present invention is no more than about
0.25° C.
[0045] The present invention provides microbial sealant
drape compositions which provide a high flashpoint for safe
use in the operating room or clinical surgery suite. The flash-
May 6, 2010
point for the disclosed microbial sealant composition is
greater than 240° F. which is greater than 100% butyl
cyanoacrylate drape products.
[0046] The present invention provides cyanoacrylate-
based microbial sealant drape compositions which form a
thin and uniform drape film on the surgical sites. The film
thickness of the disclosed cyanoacrylate liquid drape compo-
sitions was evaluated using optical microscope, which is less
than 500 pm.
[0047] The present invention provides cyanoacrylate-
based microbial sealant drape compositions which has a
desirable surface coverage. The average surface coverage of
the drape composition disclosed in the present invention
device was approximately 222.0 inch2. The average surface
coverage of the drape composition disclosed in the present
invention device is much larger than that of the commercial
applicators with the same amount of the liquid sealant.
[0048] The present invention provides cyanoacrylate-
based microbial sealant drape compositions which are com-
patible with lasers. The microbial sealant films do not ignite,
crack, blister or peel under the intense thermal energy of the
lasers so that the microbial sealant film maintains its integrity
and effectiveness as a sealant on the surgical site.
[0049] The present invention provides microbial sealant
drape compositions which are compatible with defibrillators
and electrocautery. The combined use of the disclosed micro-
bial sealant composition with defibrillator and electrocautery
does not affect the effectiveness of these devices. The dis-
closed microbial sealant composition demonstrates desirable
performance with regard to charring, plume discoloration and
cleaning of the blade upon completion of the incision and
coagulation procedures. No signs of ignition, blistering,
cracking or peeling were observed.
[0050] The present invention provides cyanoacrylate-
based microbial sealant drape films which provides more
resistance to water penetration by impact than the commer-
cial cyanoacrylate films. The microbial sealant composition
of the present invention elicited a mean value of 0.03 grams
from penetration of water by impact compared to a mean
value of 0.07 grams for butyl cyanoacrylate microbial sealant
drapes.
[0051] The present invention provides microbial sealant
drape compositions which are less irritating to the skin of a
patient than prior art compositions. Based on the skin irrita-
tion study, the primary irritation index for the disclosed
microbial sealant composition was calculated to be 0.7, while
the primary irritation index for butyl cyanoacrylate drapes
was calculated to be 1.4.
[0052] The present invention provides microbial sealant
drape compositions which are safe and biocompatible. Cyto-
toxicity, genotoxicity, local irritation after implantation, and
delay-type hypersensitivity of the disclosed microbial sealant
compositions were evaluated based on ISO 10993, which
confirmed the disclosed compositions are safe to be used as
microbial sealant to inhibit the surgical site infections.
BRIEF DESCRIPTION OF THE FIGURES
[0053] FIG. 1 is an illustration of a preferred embodiment
of the applicator for the cyanoacrylate microbial sealant
drape compositions.
DETAILED DESCRIPTION OF THE INVENTION
[0054] The present invention provides compositions com-
prising sterile mixtures of cyanoacrylates with different
US 2010/0112036 A1
length alkyl chains for use as a liquid microbial sealant drape
that can inhibit the surgical site infections. The drape film
formed upon polymerization of the mixture of cyanoacrylates
prevents the spread of microorganisms by trapping or immo-
bilizing bacteria that survive on patient’s skin after common
skin preparation procedures.
[0055] Preferred microbial sealant drape compositions dis-
closed herein include at least one cyanoacrylate with longer
alkyl chain and at least one cyanocrylate with shorter alkyl
chain. The longer alkyl chain cyanoacrylates are those con-
taining 5 or more carbon atoms in the alkyl group, which
include but are not limited to n-pentyl cyanoacrylate, iso-
pentyl cyanoacrylate, n-hexyl cyanoacrylate, iso-hexyl
cyanoacrylate, n-heptyl cyanoacrylate, 2-ethylhexyl
cyanoacrylate, n-octyl cyanoacrylate, 2-octyl cyanoacrylate,
nonyl cyanoacrylate, and decyl cyanoacrylate. The shorter
alkyl chain cyanoacrylates are those containing 4 or less
carbon atoms in the alkyl group, which include but are not
limited to methyl cyanoacrylate, ethyl cyanoacrylate, n-pro-
pyl cyanoacrylate, isopropyl cyanoacrylate, n-butyl
cyanoacrylate, isobutyl cyanoacrylate, 3-acetoxypropyl
cyanoacrylate, 2-methoxypropyl cyanoacrylate, and 3-chlo-
ropropyl cyanoacrylate.
[0056] Varying the composition ratio of cyanoacrylate with
different alkyl chains allows modification of the properties of
cyanoacrylate compositions so that bonding strength, flex-
ibility, cure time, degradability and biocompatibility can be
controlled. Cyanoacrylates with long alkyl chains lacking
oxygen-containing functional groups tend to form polymers
that degrade slowly. Compared to longer chain cyanoacry-
lates, the shorter cyanoacrylate monomers have a higher
degree of tissue toxicity due to their rapid degradation into
formaldehyde and the corresponding cyanoacetate. Polymer
films comprising longer alkyl chain cyanoacrylate tend to be
more flexible than those made of shorter alkyl chain
cyanoacrylates. Shorter alkyl chain cyanoacrylates have
advantageous properties as tissue adhesives. For example,
shorter alkyl chain cyanoacrylates provide faster curing speed
and stronger bond strength as compared to longer alkyl chain
cyanoacrylates.
[0057] In a preferred embodiment of compositions of the
present invention, liquid microbial sealant drape composi-
tions comprise 2-octylcyanoacrylate (OCA) in combination
with n-butyl cyanoacrylate (BCA). In order to investigate the
stability of the mixture of 2-octyl cyanoacrylate and n-butyl
cyanoacrylate, a series of mixed cyanoacrylate compositions
with different ratio of OCA/BCA were prepared and sub-
jected to sterilization. Sterilization of the compositions is a
requirement for their use as microbial sealants. Therefore it is
important that the compositions can be sterilized without
significant viscosity change. The compositions tested were
composed of cyanoacrylates having the following ratios of
n-butyl cyanoacrylate to 2-octyl cyanoacrylate: 10:90, 20:80,
30:70, 40:60, 50:50, 60:40, 70:30, 80:20 and 90:10. Table 1
shows the viscosity of the 2-octyl cyanoacrylate and n-butyl
cyanoacrylate mixtures before and after the E-beam steriliza-
tion. The compositions were sterilized in HDPE bottles (1
ounce). Viscosity change correlates with the stability of
cyanoacrylate monomers, stability being an important crite-
rion for selecting the appropriate cyanoacrylate mixture com-
positions for use as microbial sealants. As shown in Table 1,
E-beam sterilization has different effects on different mix-
tures of 2-octyl cyanoacrylate and n-butyl cyanoacrylate
depending on the ratio of the two cyanoacrylates. The viscos-
May 6, 2010
ity of the different compositions before the sterilization
ranges from 3.68 to 3.88 cps and the difference is within the
measurement accuracy of the viscometer. Mixed cyanoacry-
late compositions having approximately 60% to about 90%
2-octyl cyanoacrylate demonstrate slight viscosity increases
after E-beam sterilization. The preferred range of viscosity
change is approximately 0% to 200%. The viscosity of the
mixed cyanoacrylate compositions having about 50% or less
of 2-octyl cyanoacrylate increases dramatically after the
E-beam sterilization. This is indicative of instability of those
compositions after undergoing E-beam sterilization. Mixed
cyanoacrylate compositions having about 20% or less of
2-octyl cyanoacrylate cured after the E-beam sterilization and
are unsuitable for microbial sealant compositions. In a more
preferred embodiment of compositions of the present inven-
tion, compositions with about 60% to about 90% of 2-octyl
cyanoacrylate are used as the liquid microbial sealant to pre-
vent the surgical site infections. More preferably, composi-
tions with about 70% to about 90% of 2-octyl cyanoacrylate
are used. Even more preferably, compositions with about
80% of 2-octyl cyanoacrylate and about 20% of n-butyl
cyanoacrylate is used as the liquid microbial sealant to pre-
vent the surgical site infections.
TABLE 1
Viscosity ofmixed OCNBCA compositions before and after E-beam
sterilization.
Average viscosity cps
Formulation Composition Before Sterilization After Sterilization
la 1:9 BCNOCA 3.68 5.11
1b 2:8 BCNOCA 3.68 5.71
1c 3:7 BCNOCA 3.68 5.11
1d 4:6 BCNOCA 3.88 6.95
le 5:5 BCNOCA 3.88 15.77
1f 6:4 BCNOCA 3.88 68.03
lg 7:3 BCNOCA 3.68 505.63
1h 8:2 BCNOCA 3.68 Cured
11 9:1 BCNOCA 3.68 Cured
[0058] In preferred embodiments of the present invention
the liquid microbial sealant drape compositions provide at
least two-year shelf life. The stability of the sterile cyanoacry-
late adhesive compositions has evaluated by the accelerated
aging. The accelerated aging test of the mixed cyanoacrylate
microbial sealant composition was performed in an oven at
80° C. for a period of 12 days. The investigated compositions
were tested for viscosity at intervals of 3, 6, 9 and 12 days.
Based onASTM F1980-2, 12 days accelerated aging at 80° C.
correlates to 2 years of shelf life at ambient temperatures.
Table 2 shows the viscosity of a sterile microbial sealant
composition in an applicator containing 80% of 2-octyl
cyanoacrylate and 20% of n-butyl cyanaocrylate at day 0, 3, 6,
9 and 12 of the accelerated aging at 80° C. The viscosity of the
cyanoacrylate-based microbial sealant compositions
increases as the accelerated aging proceeds but the increased
viscosity of the aged samples at day 12 does not affect the
performance of liquid drape compositions nor the dispensing
of the compositions from the applicator.
US 2010/0112036 A1
TABLE 2
Viscosity of the sterile microbial sealant drape composition before
and after the accelerated aging at 80° C. for 12 days.
Viscosi c s
Test 1 Test 2 Test 3 Average
Day 0 4.29 3.68 3.06 3.68
Day 3 4.29 3.68 4.90 4.29
Day 6 4.90 4.90 5.52 5.11
Day 9 6.13 6.13 6.74 6.33
Day 12 27.0 25.7 30.6 27.8
[0059] In preferred embodiments of the present invention
the microbial sealant drape composition is packaged in a
user-friendly, single use applicator. As shown in FIG. 1, the
applicator comprises a compartment containing the mixed
cyanoacrylate compositions and a sponge applicator tip
through which the liquid drape compositions may be applied
to the surgical site. The applicator compartment is preferably
air and water tight with a sealing mechanism to prevent con-
tamination to the mixed cyanoacrylate monomers inside.
When the compartment is opened, the mixed cyanoacrylate
liquid sealant is evenly distributed onto the sponge applicator
tip. Cyanoacrylate liquid drape compositions can be easily
dispensed onto the sponge from an applicator once the sponge
connection is folded. A uniform sealing film is formed by
applying the cyanoacrylate-saturated sponge tip onto surgical
sites.
[0060] According to the present invention, the microbial
sealant drape compositions of the present invention can effec-
tively reduce the amount of microorganisms in the surgical
site. In preferred embodiments the microbial sealant compo-
sitions are at least 99.9% effective in preventing the spread of
the clinically relevant bacteria on the surgical sites under a
variety of usage conditions. The in vitro immobilization of
microorganisms by the microbial sealant compositions was
evaluated using sterile pig skin incised with a sterile surgical
scalpel. Microorganisms used to challenge the surgical site
may include without limitation pathogenic gram negative
bacteria, gram positive bacteria, yeast and Corynebaczerium
sp. The immobilization of microorganisms by the microbial
sealant compositions of the present invention was evaluated
under different conditions which included without limitation
using the microbial sealant composition without incision,
using the microbial sealant composition with incision, using
the microbial sealant composition with incision and skin sur-
gical preps, and using the microbial sealant composition with
incision and surgical incise drapes.
[0061] The microbial sealant drape compositions of the
present invention were effective in preventing the mitigation
in the test organism on the surgical site. Complete effective-
ness was manifest as greater than 3 .9 log 10 mitigation in the
case of S. epidermidis, MRSA, Corynebaczerium species,
Pseudomonas aeruginosa and greater than 4 log 10 mitiga-
tion for Candida albicans. The microbial sealant composi-
tions do not have an adverse effect on the effectiveness of
surgical preps. The microbial sealant compositions do not
need to be used in combination with an antimicrobial surgical
incise drape. Instead the microbial sealant compositions of
the present invention may be used as a substitute for an
antimicrobial surgical incise drape.
May 6, 2010
[0062] According to the present invention, the preferred
microbial sealant drape compositions can reduce microbial
colonization by at least 99.9% within 15 minutes of applica-
tion and maintain at least a 99. 9% reduction throughout the 24
hours post treatment
[0063] According to the present invention, the microbial
sealant drape compositions are resistant to the passage of
blood-borne pathogens. Testing based on ASTM F1671 “Test
Method for Resistance of Materials Used in Protective Cloth-
ing to Penetration by Blood-Bome Pathogens Using Phi-
X174 Bacteriophage Penetration as a Test System” was con-
ducted to demonstrate the pathogen resistance. The test
results indicated that the disclosed microbial sealant film is
resistant to the passage of blood-borne pathogens using a
viral penetration as a test system.
[0064] Preferred microbial sealant drape compositions of
the present invention generate less heat during the application
compared to commercially available drape products. Poly-
merization of cyanoacrylate is an exothermic process. The
amount of heat released during polymerization is related to
the length of the alkyl chain of the cyanoacrylate. Cyanoacry-
lates with shorter length chains release more heat. Too much
heat generated from the application of cyanoacrylates onto
human skin makes the patient uncomfortable. The exother-
mic effect of microbial sealant product of the present inven-
tion and a commercially available product was evaluated
using an Infrared Thermometer to measure the temperature
change on human skin. The skin temperature of patients was
measured before and after the application of the drape prod-
ucts onto human skin. The average temperature increase after
applying microbial sealant composition disclosed in the
present invention and a commercially available product were
0.25 and 0.41 ° C., respectively. The test results indicate that
less heat is released from the application of the preferred
composition disclosed in the present invention than that of a
commercially available product.
[0065] According to the present invention, the microbial
sealant drape compositions of the present invention have a
high flashpoint for safe use in the operating room or clinical
surgery suite. The test was performed in accordance with
ASTM D56-05, ISO 3679:2004 determination of flashpoint-
rapid equilibrium closed cup method. The flashpoint for the
microbial sealant compositions of the present invention is
greater than 240° F. The flashpoint of the 100% butyl
cyanoacrylate microbial sealant compositions is about 227°
F
[0066] According to the present invention, no cyanoacry-
late residue was detected on a surgical blade through which an
incision was made on a substrate covered with a microbial
sealant drape compositions disclosed herein. The detection
limit of the test was 5 ppm. The residue analysis on a surgical
blade confirmed that no detectable cyanoacrylate sealants
were transferred into the incision wound site.
[0067] According to the present invention, the preferred
microbial sealant drape compositions of the present invention
provide a desirable degradation profile. The integrity (degra-
dation over time) of the disclosed microbial sealant film was
evaluated following topical application to the skin of three
pigs and comparisons were made to commercially available
drapes. The microbial sealant films applied at each applica-
tion site were evaluated for degradation at approximately 8
hours after application, and 1, 2, 3, 4, 6, 8, 10, 12, 14, and 16
days after application. Degradation of both the disclosed
microbial sealant film and the commercial product was evi-
US 2010/0112036 A1
dent by the first observation interval (8 hours after applica-
tion). At this time, 2 out of 12 test sites with the disclosed
microbial sealant film remained intact and 5 out of 12 sites
with the predicate device were intact. When the study ended
on day 16, the microbial sealant film of the present invention
was partially present in 2 of the 12 sites, while the commercial
product was absent from all 12 sites.
[0068] According to the present invention, the liquid
microbial sealant compositions are compatible with currently
available skin preparation products, surgical incise drape
products and wound closure products. Compatibility with
current products means that the application of the disclosed
microbial sealant drape composition does not adversely
affect the performance of wound closure products and surgi-
cal incise drapes. Skin preparation products that may be used
in concert with the compositions of the present invention
include without limitation Chloraprep, Duroprep, 10% Povi-
done iodine and Betadine. Duraprep is preoperative skin
preparation product comprising iodine povacrylex and iso-
propyl alcohol. ChloraPrep is a rapid-acting, persistent, and
broad- spectrum preoperative skin preparation product, which
consists of 2% Chlorhexidine Gluconate in 70% isopropyl
alcohol. Betadine is a consumer-available topical antiseptics
containing 10% of povidone-iodine. Surgical incise drapes
may also be used with the cyanoacrylate compositions of the
present invention including without limitation 3M Steri-strip
and loban 2. Steri-Strip is an antimicrobial skin closure prod-
uct that is made of a porous, non-woven backing coated with
a pressure-sensitive adhesive which contains iodophor and is
reinforced with polyester filaments for improved strength.
loban 2 is an antimicrobial surgical incise drape with an
iodophor impregnated adhesive providing a sterile surface
and antimicrobial activity throughout the procedure. The
compatibility of the preferred liquid microbial sealant com-
positions with current commercial products used for prevent-
ing surgical site infections was investigated by observing the
effect of the disclosed liquid drape product on the adhesion
property of surgical incise drape in the absence and presence
of different skin preparation products.
[0069] The liquid cyanoacrylate sealant compositions of
the present invention are also compatible with currently avail-
able wound closure products. The wound closure products
may include SurgiSeal, Dermabond and Steri-Strip. Derrna-
bond is a liquid bonding adhesive that holds cuts, incisions
and wounds together. SurgiSeal is cyanoacrylate-based topi-
cal skin adhesive for the closure of wound and incisions to
provide a flexible, water-resistant, antimicrobial protective
coating, which provides the optimal balance between bond
strength and flexibility.
[0070] According to the present invention, the preferred
liquid cyanoacrylate sealant drape composition is compatible
with lasers. The lasers that may be used in concert with the
compositions of the present invention include without limi-
tation CO2, Nd:YAG, and Diode. The disclosed microbial
sealant is intended to be used after typical operative skin
preparation prior to a surgical incision. Lasers may be
required to be used for skin incision, ablation, or coagulation
for a surgical procedure. The in vitro study was conducted to
evaluate the effect of both free beam and contact use of the
lasers on the disclosed microbial sealant film formed on pig
skin. The combined use of a skin prep such as Betadine with
the disclosed microbial sealant composition was also inves-
tigated using a Diode laser. The integrity of the disclosed
microbial sealant film was evaluated by macroscopic obser-
May 6, 2010
vations for cracking, blistering and peeling. The intense ther-
mal energy of the lasers was used to determine if the disclosed
microbial sealant film would ignite. The results showed that
the disclosed microbial sealant film did not ignite, crack,
blister or peel for all three laser types when used with either
free beam or contact thermal energy applications so that the
microbial sealant maintains its integrity and effectiveness as a
sealant for the surgical procedure. These same results were
obtained when combined with the surgical skin preparation
product when the Diode laser was used with either free beam
or contact laser.
[0071] According to the present invention, the cyanoacry-
late-based microbial sealant compositions are compatible
with defibrillators and an electrocautery. The in vitro study
was conducted on porcine skin to evaluate the effect of the
microbial sealant compositions of the present invention on
the performance of the defibrillator and electrocautery. The
microbial sealant composition was applied onto porcine skin.
A metal plate or probe was attached on the underneath side of
the porcine skin to measure the voltage of the defibrillator. In
order to evaluate the compatibility with Electrocautery, a
commercially available Electrocautery device was used to
make incisions and coagulations on the porcine skins covered
with the disclosed microbial sealant film. The Electrocautery
settings were made at 70 watts for both incision and coagu-
lation. The single coat application of the microbial sealant
compositions of the present invention did not significantly
decrease the conductance of the energy being discharged
from the defibrillator. There was no observation of ignition,
blistering, cracking or peeling. When used with the electro-
cautery, the microbial sealant compositions demonstrated
desirable performance with regard to charring, plume discol-
oration and cleaning of the blade upon completion of the
incision and coagulation.
[0072] According to the present invention, the cyanoacry-
late liquid drape compositions provide a thin and uniform film
on the surgical sites. In preferred embodiments of the present
invention the drape film has a thickness of from about 5 to
about 400 pm. More preferably, the drape film provides a
thickness of about 10 to 200 pm, more preferably from about
30 to 80 um and still more preferably from about 50 to 60 pm.
The film thickness study indicates the formation of thin and
uniform films of the disclosed liquid drape compositions.
[0073] According to the present invention, the liquid
microbial sealant drape compositions provide greater resis-
tance to penetration of water by impact than the commercially
available liquid drapes. The resistance of the microbial seal-
ant compositions of the present invention to the penetration of
water by impact was investigated according to the American
Association of Textile Chemists and Colorists (AATCC) test
method. A volume of water is allowed to spray against the taut
surface of the disclosed microbial sealant film backed by a
weighted blotter. The blotter was then reweighed to determine
water penetration. The microbial sealant compositions of the
present invention have an average value of 0.03 grams from
penetration of water by impact compared to an average value
of 0.07 grams for a commercial drape composition. The test
results indicate that the disclosed microbial sealant composi-
tion provides twice more resistance to water penetration by
impact than the commercial product.
[0074] According to the present invention, the cyanoacry-
late liquid sealant drape compositions are safe and effective as
a surgical sealant product useful for inhibiting surgical site
infections. The safety and biocompatibility of the disclosed
US 2010/0112036 A1
liquid drape composition has been evaluated based on the
International Organization for Standardization (ISO) 10993,
Biological Evaluation of Medical Devices. Cytotoxicity was
measured on the preferred liquid microbial sealant composi-
tion using an in vitro biocompatibility study. The liquid
microbial sealant compositions of the present invention are
not cytotoxic. For comparison, the in vitro cytotoxicity of
prior art device was also evaluated, which showed no evi-
dence of causing cell lysis or toxicity.
[0075] According to the present invention, the preferred
liquid cyanoacrylate-based microbial sealant drape composi-
tion is less irritating than the prior art device, which was
confirmed by the primary skin irritation study and ISO intra-
cutaneous study.
[0076] According to the present invention, the preferred
liquid microbial sealant drape composition is not genotoxic.
Bacterial reverse mutation test, mouse peripheral blood
micronucleus study and in vitro chromosomal aberration
study in mammalian cells confirmed that the compositions
are not genotoxic.
[0077] The mixed cyanoacrylate compositions may be sta-
bilized with a combination of free radical stabilizer and
anionic stabilizer. In embodiments of the present invention,
the preferred primary free radical stabilizer is butylated
hydroxyl anisole (BHA). BHA is used in an amount of about
200 to about 15000 ppm, preferably about 1000 to about
10000 ppm, more preferably about 2000 to about 8000 ppm.
Other free radical stabilizers that may be used include without
limitation, hydroquinone; catechol; hydroquinone monom-
ethyl ether and hindered phenols such as butylated hydroxya-
nisol; 4-ethoxyphenol; butylated hydroxytoluene (BHT, 2,6-
di-tert-butyl butylphenol), 4-methoxyphenol (MP);
3-methoxyphenol; 2-tert-butyl-4methoxyphenol; 2,2-meth-
ylene-bis-(4-methyl-6-tert-butylphenol).
[0078] In embodiments of the present invention, the pre-
ferred primary anionic stabilizer is sulfur dioxide in an
amount of about 2 to about 500 ppm, preferably from about
10 ppm to about 200 ppm. The anionic stabilizer may be a
very strong acid including without limitation perchloric acid,
hydrochloric acid, hydrobromic acid, toluenesulfonic acid,
fluorosulfonic acid, phosphoric acid, ortho, meta, or para-
phosphoric acid, trichloroacetic acid, and sulfuric acid. The
very strong acid may be used in an amount of 0 to about 250
ppm, preferably from about 5ppm to 50 ppm. Preferably, the
very strong acid stabilizer is sulfuric acid, phosphoric acid or
perchloric acid.
[0079] According to the present invention, the cyanoacry-
late-based microbial sealant drape compositions are sterilized
for medical use. The sterilization can be accomplished by
common techniques, and is preferably accomplished by
methods including, but not limited to, chemical, physical, and
irradiation methods. An example of a chemical method
includes, but is not limited to, exposure to ethylene oxide.
Examples of irradiation methods include, but are not limited
to, gamma irradiation, electron beam irradiation (E-beam),
and microwave irradiation.
[0080] In preferred embodiments of the present invention,
E-beam is used to sterilize the cyanoacrylate-based microbial
sealant compositions. The dose of E-beam irradiation applied
should be sufficient enough to sterilize both the package and
the adhesive inside. The E-beam irradiation may be in a
dosage of from about 5 to 50 kGy, and more preferably from
about 12 to about 25 kGy. E-beam irradiation is preferably
conducted at ambient atmosphere conditions and the expo-
May 6, 2010
sure time to the irradiation is preferably from about 1 to about
60 seconds, more preferably from about 10 seconds to 60
seconds.
[0081] In preferred embodiments of the present invention,
the viscosity of the preferred cyanoacrylate-based microbial
sealant composition changes upon the E-beam sterilization.
The average viscosity of the preferred microbial sealant drape
composition comprising 20% butyl cyanoacrylate and 80%
octyl cyanoacrylate before sterilization is 3.68 cps. After the
sealant composition is subjected to E-beam sterilization the
viscosity was measured to be 5.71 cps. The prior art refer-
ences indicate that E-beam sterilization can induce serious
partial polymerization of cyanoacrylate, which would lead to
a large increase in viscosity.
[0082] In order to reduce the bioburden, the cyanoacrylate-
based microbial sealant drape compositions may be filtered
through a 0.2 pm filter. The applicators with the overpack may
also be sterilized with heat, ethylene oxide prior to the final
E-beam irradiation.
[0083] The sterility of the cyanoacrylate-based microbial
sealant drape compositions may be analyzed by Bacteriosta-
sis and Fungistasis tests. In embodiments of the present
invention, a Sterility Assurance Level (SAL) should be
obtained at a minimum of 10‘3 , which means that the prob-
ability of a single unit being non-sterile after sterilization is 1
in 1000. In more preferred embodiments, the Sterility Assur-
ance Level may be at least 10‘6.
[0084] The following non-limiting examples are intended
to further illustrate the present invention.
EXAMPLE 1
Setting Time Measurement
[0085] Pig skin (4>< MEM) with 5% serum and 2% antibi-
otics. The test extract was placed onto three separate mono-
US 2010/0112036 A1
layers of L-929 mouse fibroblast cells propagated in 5% CO2.
High density polyethylene was used as the negative control
and tin stabilized polyvinylchloride was used as the positive
control. All monolayers were incubated at 37° C. in the pres-
ence of 5% CO2 for 48 hours, which was then examined
microscopically to determine any change in cell morphology.
The liquid microbial sealant compositions of the present
invention did not cause cell lysis or toxicity.
EXAMPLE 8
Genotoxicity Study 1
[0092] A glass rod was cleaned with 70% isopropyl alcohol
and allowed to air dry. The rod was then coated with a micro-
bial sealant drape composition comprising 80% of 2-octyl
cyanoacrylate and 20% of n-butyl cyanoacrylate up to 4 cm
and allowed to dry for at least 1 minute prior to extraction with
dimethyl sulfoxide (DMSO) and 0.9% sodium chloride at 37°
C. for 72 hours. Another glass rod without cyanoacrylate
liquid drape was similarly subjected to the extraction condi-
tions for use as a negative control. Known mutagens, benzo
[a]pyrene and 2-nitrofluorene, were used as positive control
to demonstrate that tester strain TA 98 was sensitive to muta-
tion reversion to wild type. For tester strains TA100 and TA
1535, sodium azide and 2-aminoanthracene were used as
positive controls. For tester 1537, 2-aminoanthracene and
ICR-191 were used as positive controls. For tester strain
WP2uvrA, 2-aminoanthracene and methylmethane-sul-
fonate were used as positive controls.
[0093] Tubes containing molten top agar supplemented
with tryptophan for the Escherichia coli or with histidine-
biotin solution for the Salmonella zyphimurium were inocu-
lated with culture for each of the five tester strains and with
the DMSO and saline extracts of the disclosed cyanoacrylate
liquid drape film. Sterile water for injection (SW1) or S9
homogenate simulating metabolic activation was added as
necessary. Trytophan-free media plates (for E. coli) and his-
tidine-free media plates (for S. zyphimurium) were prepared
in triplicate as follows: 1) DMSO and saline extracts of the
cyanoacrylate liquid drape film with and without S9 activa-
tion; 2) negative controls with and without S9 activation; and
3) positive controls with different tester strains in the absence
and presence of S9 activation.
[0094] The plates were incubated at 37° C. for 2 to 3 days.
Following the incubation period, the revertant colonies on
each plate were recorded. The mean number of revertants and
standard deviation was determined. The mean number of
revertants of the test plates was compared to the mean number
of revertants of the negative control for each of the five tester
strains. It was concluded that, under the study conditions, the
disclosed liquid microbial sealant compositions in both
DMSO and saline extracts were not mutagenic to Salmonella
Zjzphimurium strains (TA98, TA100, TA1535, and TA1537),
and were not mutagenic to tryptophan-dependent Escheri-
chia coli strain WP2uvrA.
EXAMPLE 9
Genotoxicity Study 11
[0095] A glass rod was cleaned with 70% isopropyl alcohol
and allowed to air dry, and then coated with the microbial
sealant drape compositions comprising 80% of 2-octyl
cyanoacrylate and 20% of n-butyl cyanoacrylate up to 4 cm.
The drape was allowed to dry for at least 1 minute prior to
May 6, 2010
extraction with dimethyl sulfoxide (DMSO) and 0.9%
sodium chloride at 37° C. for 72 hours. Additional test rods
without cyanoacrylate microbial sealant were subjected to the
same extraction conditions as the test article and were used as
negative controls. Methyl methanesulfonate (MMS) in saline,
an antineoplastic drug known to have mutagenic properties,
was used as a positive control.
[0096] Five groups of mice, each of which consisted of 6
male and 6 female, were injected with cyanoacrylate liquid
drape in SC extract, cyanoacrylate liquid drape in SO extract,
negative control in SC, negative control in SO, and positive
control with methyl methanesulfonate, respectively. Each
mouse received an intraperitoneal injection at a dose of 20
ml/kg of the appropriate extract accordingly for consecutive
three days.All animals were observed immediately following
injection and on a daily basis to access general health. On day
4, blood was collected from the tail veins of each mouse and
solutions were prepared. The normochromatic erythrocytes
were evaluated for the presence of micronuclei. The fre-
quency of micronucleated reticulocytes (MN-RETs) was
determined and used as an index of genotoxicity. The fre-
quency of reticulocytes relative to total erythrocytes was cal-
culated as an indication of stem cell toxicity. Both SC and SO
extracts of the cyanoacrylate liquid drapes of the present
invention did not show statistically significant increases in the
frequency of MN-RETs. Cyanoacrylate liquid microbial
sealant compositions of the present invention are not geno-
toxic under the study conditions. Also, there was no evidence
of cellular toxicity from extracts of the disclosed cyanoacry-
late liquid drape composition.
EXAMPLE 10
Local Irritation and Toxicity Study
[0097] Local irritation or toxicity effect after implantation
of the microbial sealant drape compositions comprising 80%
of 2-octyl cyanoacrylate and 20% of n-butyl cyanoacrylate
was conducted to evaluate the potential for a local irritant or
toxic response to the drape implanted in direct contact with
muscle tissue. High density polyethylene was used as the
negative control. Three Albino New Zealand rabbits were
used for the test. One incision was made on each side of the
rabbit back. The fascia was cut to expose the paravertebral
muscle. A pocket was formed with a hemostat between the
muscle fibers into which the implant material was introduced.
After four weeks, the rabbits were weighed and then eutha-
nized by an intravenous injection of a sodium pentobarbital
based drug. The paravertebral muscles were dissected free
and fixed in 10% neutral buffered formalin to facilitate cut-
ting. The tissue was macroscopically examined using low
magnification to look for capsule formation or other signs of
irritation. The excised sections were also histologically pro-
cessed for microscopic evaluations. The disclosed microbial
sealant drapes of the present invention caused no macro-
scopic reaction under the study conditions, while micro-
scopic examination indicated the disclosed composition was
moderately irritating to the tissue.
EXAMPLE 11
ISO lntracutaneous Study
[0098] lntracutaneous study of microbial sealant drape
compositions comprising 80% of 2-octyl cyanoacrylate and
20% of n-butyl cyanoacrylate was conducted to determine
US 2010/0112036 A1
whether leachables extracted from the disclosed microbial
sealant composition wound cause local dermal irritant effects
following injection into rabbit skin. The glass rods were
wiped clean with 70% isopropyl alcohol and allowed to air
dry. The glass rod was coated with the disclosed microbial
sealant compositions up to 4 cm and allowed to air dry for at
least one minute prior to placing in the extraction container.
The test article was extracted in 0.9% sodium chloride USP
solution (SC) and sesame oil, NF 9 (S0) at 37° C. for 72
hours. A 0.2 ml dose of the test article extract was injected by
the intracutaneous route into five separate sites on the right
side of the back of each rabbit. Injections were spaced
approximately 2 cm apart. The appearance of each injection
site was noted immediately after injection. Observations for
erythema and edema were conducted at 24, 48, and 72 hours
after injection. Under the conditions of this study, there was
no erythema and no edema from the SC extract injected
intracutaneously into rabbits. There was very slight erythema
and very slight edema from the SO extracts injected intracu-
taneously into rabbits.
EXAMPLE 12
ISO Skin Irritation Study
[0099] Skin irritation study of cyanoacrylate-based micro-
bial sealant drape compositions comprising 80% of 2-octyl
cyanoacrylate and 20% of n-butyl cyanoacrylate was con-
ducted to evaluate the potential for a single topical application
of the disclosed microbial sealant composition to irritate skin.
New Zealand white male rabbits were used for this study. On
the day of treatment, four sites, two on each side of the back
cranially and caudally, were designated on each rabbit. A 0.5
ml portion of the disclosed microbial sealant composition
was applied topically to each cranial site by introduction
under a 4 ply gauze layer to an area of skin approximately 25
mm>99% effective in preventing the
spread of the microorganisms into the wound site.
EXAMPLE 19
In vivo Bacteria Immobilization
[0109] A total of 60 healthy volunteers (29 females and 31
males) were recruited to evaluate the in vivo bacteria immo-
bilization of a microbial sealant composition comprising 20%
butyl cyanoacrylate and 80% octyl cyanoacrylate. The study
included a 14-day pretreatment washout period for stabiliza-
tion of skin bacteria flora. During the washout period, sub-
jects refrained from using any topical antimicrobials, sys-
temic antibiotics, medical soaps, lotions, shampoos, etc, for at
least two weeks before the evaluation and throughout the
study. The tested area consisted of the right inguinal region.
May 6, 2010
Mean difference
score (test — control)
Very slight edema (barely perceptible)
Well-defined edema (edges ofarea
well-defined by definite raising)
Severe edema (raised more than 1 mm,
and extending beyond exposure area)
Hair was removed using a sterile disposable clipper device. A
sterile drape was used to isolate the inguinal area from the rest
of the body and then a surgical marker was used to draw four
different 1 inch squares separated by 1 inch of normal skin in
which the microbial sealant composition was applied. Using
sterile gloves the products were applied onto the skin in its
designated areas and allowed to dry. Sterile gauze was placed
over the test area to avoid sub sequent contamination.
Swabbed samples from skin were collected at 15 minutes, 4
hours and 24 hours after the initial application of the micro-
bial sealant composition. The sample collection procedure
was performed using a sterile technique including sterile
gloves, sterile microbial sealants, surgical masks and hats.
After the sampling was completed the entire contents of the
tube was poured carefully onto a 1 mL Petrifilm aerobic plate
(plate count agar) and the plates were incubated for 48 hours
at 30° C. 3MTM PetrifilmTM plate was used to quantify colony
counts. At 15 minutes, the absolute log reduction was 5.568
for the disclosed microbial sealant composition. The absolute
log reduction of bacteria for the disclosed microbial sealant
composition is 4.299 and 3 .33 at 4 hours and 24 hours, respec-
tively.
EXAMPLE 20
In vitro Chromosomal Aberration Study in Mamma-
lian Cells
[0110] A chromosomal aberratioin study was conducted to
determine whether an extract of the microbial sealant drape
composition wound cause clastogenic changes in Chinese
Hamster Ovary (CHO). A glass rod was sterilized with 70%
isopropyl alcohol and allowed to air dry. The glass rod was
then coated (4 cm) with a microbial sealant composition
comprising 20% butyl cyanoacrylate and 80% octyl
cyanoacrylate and allowed to air dry for at least 1 minute prior
to placing the coated rod in the extraction container. A single
preparation was extracted with DMSO with agitation at 37°
C. for 72 hours. Following extraction, the DMSO extract was
diluted with McCoy’s 5A medium to a final concentration of
25% prior to testing. Aveclor 1254:induced rat liver (S9
homogenate) was used as metabolic activation. The S9 homo-
genate is prepared from male, Sprague Dawley rats. An
US 2010/0112036 A1
uncoated glass rod was subjected to the same extraction con-
ditions to serve as a negative control. A known direct acting
genotoxic compound, Mitomycin C (MMC), was used as a
positive control to demonstrate that CHO cells were sensitive
to mutagens in the absence of metabolic activation. The
microbial sealant composition extract, negative control, and
positive control were tested in triplicate. For the assays con-
ducted without metabolic activation, the growth medium in
each of three test culture flasks was replaced with 10 ml of the
prepared extracts. For the assay conducted with metabolic
activation, the test samples were supplemented with isocitrate
dehydrogenase (NADP+) at 60 111/ ml and S9 at 20 p.l/ml.After
18 hours of incubation at 37° C. in the presence of CO2, the
medium was decanted and the cultures were rinsed twice with
4-6 ml of calcium magnesium free phosphate buffered saline
(CMF-PBS). The flasks were incubated for an additional 2
hours at 37° C. After harvesting, slides of the cells were
prepared, stained with Giemsa, and examined microscopi-
cally for chromosomal aberrations at 100>< magnification.
Under the conditions of this assay, the DMSO test extract of
the disclosed microbial sealant composition was not consid-
ered genotoxic to Chinese Hamster Ovary cells in the absence
of S9 metabolic activation. The prepared McCoy’s extract
was not considered genotoxic to Chinese Hamster Ovary cells
in the presence or absence of S9 metabolic activation. The
positive and negative controls performed as expected.
EXAMPLE 21
Resistance to Impact Penetration
[0111] The resistance of a microbial sealant drape compo-
sition comprising 20% butyl cyanoacrylate and 80% octyl
cyanoacrylate to the penetration of water by impact was
evaluated by following the American Association of Textile
Chemists and Colorists (AATCC) test method. Test sample
films of the microbial sealant composition were made that
measured 178x230 mm. The samples and the blotting paper
were conditioned in an atmosphere of 65:2% relative humid-
ity (RH) at 21:1° C. for 4 hours before testing. After clamping
the film onto an inclined stand, a standard blotter 152x230
mm was weighed and inserted beneath the test sample. A
500110 ml volume of distilled water at 27:1° C. was poured
into a funnel of the tester and allowed to spray onto the test
sample of the microbial sealant composition. After the spray-
ing, the test sample was carefully lifted, the blotter removed
and reweighed to determine the amount of water that pen-
etrated the film during the test. The mean value for the micro-
bial sealant composition was 0.03 grams. Under the same
conditions a commercial microbial sealant film comprised of
100% butyl cyanoacrylate displayed a mean value of 0.07
grams.
EXAMPLE 22
Sealant Film Integrity Over Time
[0112] The integrity (degradation over time) of a microbial
sealant composition comprising 20% butyl cyanoacrylate and
80% octyl cyanoacrylate was evaluated following topical
application to the skin of three pigs and compared to another
microbial sealant product. The pigs were restrained in a sling
for up to 30 minutes during the application procedures. To
reduce possible stress at being restrained in the sling, the pigs
were initially conditioned to the sling over the course of 3
days prior to commencement of the application procedures.
May 6, 2010
The day prior to treatment, each pig was weighed and placed
in a sling. The hair on the dorso-lateral area was removed. The
depilated skin was washed with povidone iodine scrub, rinsed
well with water, and dried. On the day of the application
procedure, each pig was placed in a sling. The depilated area
of the back was scrubbed with povidone iodine, wiped with
70% isopropyl alcohol andpainted with 10% povidone iodine
antiseptic. The microbial sealant composition of the present
invention and another commercial drape were applied to four
sites approximately 1>X< * >X< *
Coments go here:
- Log in to post comments