��������������(19) United States
(12) Patent Application Publication (10) Pub. No.: US 2008/0241249 A1
US 20080241249A1
Quintero et al. (43) Pub. Date: Oct. 2, 2008
(54) CYANOACRYLATE COMPOSITE Publication Classification
(51) Int. Cl.
. . . A61K 9/00 (2006.01)
(75) Inventors: Julian A. Qulntero, Rale1gh,l\IC A61K 31/78 (2006.01)
(US); Jerry Y- Jonn, Shanghal A61P 1 7/00 (2005.01)
(CN) (52) U.S. Cl. .................................... .. 424/487; 424/7802
(57) ABSTRACT
C01Te5P011de11Ce Address? An adhesive composite composition is provided including
(73)
(21)
(22)
BRINKS, HOFER, GILSON & LIONE
2801 SLATER ROAD, SUITE 120
MORRISVILLE, NC 27560 (US)
Assignee:
Appl. No.:
Filed:
PS1)
\/
Modulus
16000
14000
12000
10000
8000
6000
4000
2000
Closure Medical Corporation,
Raleigh, NC (US)
11/731,839
Mar. 30, 2007
one or more polymerizable monomers and one or more metal
stearates. The one or more polymerizable monomers may be
a cyanoacrylate monomer. The adhesive composite compo-
sition may further comprise a plasticizer, an initiator, a rate
modifier, a stabilizer, a colorant, a heat dissipating agent, or
other additives. Methods for the application of the adhesive
composite compositions to living tissue are also provided.
The adhesive composite composition provides an adhesive
composite material upon polymerization which is a polymer
matrix entrapping the metal stearate. Polymerization of the
adhesive composite composition at a site on living tissue
provides an adhesive composite material which promotes
microcirculation and tissue growth at the site of application of
the adhesive composite composition.
Cyanoacrylaie Composite — Modulus
Key to Composition
1. 55% CA 1 15% DBS/
30% MgSt
2. 55% CA / 15% DBS/
30% Cast
3. 65% CA/15% DBS/
20% Mgst
4. 65% CA/15°/5 DBS/
20% Cast
5. 65% 20CA/15°/o DBS/
20% CaS1
3
4 5 6
Composition
7
5. 75% CA 1 15% DBS/
10% MgSi
7. 75% CA1 15% DBS/
8 9 10% CaSi
8. 35% CA / 15% DBS
9. 100% CA
Patent Application Publication Oct. 2, 2008 Sheet 1 of 2 US 2008/0241249 A1
Cyanoacrylate Composite - Modulus
Key to Composition
1. 55% CA/ 15% DBS/
30% MgSt
2. 55% CA/ 15% DBS I
30% CaSt
3. 65% CA / 15% DBS/
20% MgSt
4. 65% CA / 15% DBS/
20% CaSt
5. 65% 20CA/ 15% DBS /
20% CaSt
6. 75% CA/ 15% DBS/
10% MgS1
7. 75% CA/ 15% DBS /
10%CaSt
1 3 3 4 5 5 7 8 9 8. 85%CA/15%DBS
Composition
FIG. 1
9. 100% CA
Cyanoacrylate Composite — Elongation at Break
Key to Composition
1. 55%CA/15% DBS/
30% MgS1
2. 55%CA/15% DBS/
30% CaSt
3. 65% CA I 15% DBS/
20°/o
4. 65% CA / 15°/o DBS/
20% CaSt
5. 65% 200A / 15% DBS/
20% CaSt
6. 75% CA/ 15% DBS/
10% Mgst
7. 75% CA/ 15% DBS/
1 2 3 4 5 6 7 8 9 10%CaSt
0 0
Composition 8. 85/OCA/15/ODBS
FIG. 2
Elongation at Break (in)
9. 100% CA
Patent Application Publication
Break Stress (PSI)
1400
1200
1000
800
600
400
200
Oct. 2, 2008 Sheet 2 of 2
US 2008/0241249 A1
Cyanoacrylate Composite — Break Stress
1
2
3
4 5 6 7 8
Composition
FIG. 3
9
9.
Key to Composition
. 55% CA / 15°/o DBSI
30% Mgst
55% CA/15% DBS/
30% CaSt
65% CA/ 15% DBSI
20% MgSt
65% CA/15% DBS/
20°/o CaSt
65% 20CA / 15% DBS/
20% CaSt
75% CA/15% DBSI
10% MgSt
75% CA/ 15% DBS/
10% Cast
85% CA/ 15% DB8
100% CA
US 2008/024l249 Al
CYANOACRYLATE COMPOSITE
BACKGROUND
[0001] 1. Field
[0002] The invention relates to adhesive composite or
matrix materials, and to their use for industrial and medical
applications.
[0003] 2. State oftheArt
[0004] Monomer and polymer adhesives are used in both
industrial (including household) and medical applications.
Included among these adhesives are the l ,l-di sub stituted eth-
ylene monomers andpolyrners, such as the or-cyanoacrylates.
Since the discovery of the adhesive properties of such mono-
mers and their resulting polymers, they have found wide use
due to the speed with which they cure, the strength of the
resulting bond formed, and their relative ease of use. These
characteristics have made or-cyanoacrylate adhesives the pri-
mary choice for numerous applications such as bonding plas-
tics, rubbers, glass, metals, wood, and, more recently, bio-
logical tissues.
[0005] Polymerizable l,l-disubstituted ethylene mono-
mers, and adhesive compositions comprising such mono-
mers, are disclosed, forexample, in U.S. Pat. No. 5,328,687 to
Leung et al. Suitable methods for applying such compositions
to substrates, and particularly in medical applications, are
described in, for example, U.S. Pat. Nos. 5,928,611; 5,582,
834; 5,575,997; and 5,624,669, all to Leu11g et al.
[0006] Medical applications of l,l-disubstituted ethylene
adhesive compositions include use as an alternate or an
adjunct to surgical sutures and staples in wound closure as
well as for covering and protecting surface wormds such as
lacerations, abrasions, bums, stomatitis, sores, and other sur-
face wounds. When an adhesive is applied, it is usually
applied in its monomeric form, and the resultant polymeriza-
tion gives rise to the desired adhesive bond.
[0007] A need exists for cyanoacrylate adhesive composi-
tions with enhanced properties for use in medical applica-
tions. Such properties include suitable viscosity, biocompat-
ibility, absorbability, flexibility and stability.
SUMMARY
[0008] An adhesive composite material is provided com-
prising a polymer matrix comprising one or more biocompat-
ible cyanoacrylate polymers and a plasticizer, and at least one
metal stearate entrapped in the polymer matrix, wherein the at
least one metal stearate is present in an amount of at least 10%
by weight of the adhesive composite material.
[0009] The adhesive composite material may further com-
prise one or more of stabilizing agents, preservatives, heat
dissipating agents, colorant, or combinations thereof. The
metal stearate may be calcium stearate, magnesium stearate
or aluminum stearate.
[0010] In an embodiment, an adhesive composite compo-
sition is provided comprising one or more biocompatible
cyanoacrylate monomers, about I to about 20 wt. % of plas-
ticizer, and greater than about 10 wt. % metal stearate. The
metal stearate may provide enhanced viscosity and may serve
to initiate polymerization of the polymerizable cyanoacrylate
monomers. When used in a patie11t’s body, the resulting poly-
merized adhesive compo site material may comprise a porous,
elastic and flexible polymer matrix.
[0011] In another embodiment, a system for treating living
tissue is provided comprising a first reservoir containing a
Oct. 2, 2008
biocompatible polymerizable cyanoacrylate monomer com-
position, a second reservoir in a r1on-contacting relationship
with the first reservoir containing a metal stearate, and an
applicator capable of combining the biocompatible polymer-
izable cyanoacrylate monomer composition and metal stear-
ate to form an adhesive composite composition and then
applying the adhesive composite composition to living tissue.
[0012] In an embodiment, a method of treating living tissue
is provided comprising providing a polymerizable monomer
composition comprising one or more biocompatible poly-
merizable cyanoacrylate monomers, providing a metal stear-
ate, mixing the polymerizable monomer composition and
metal stearate to form a biocompatible adhesive composite
composition comprising a suspension of the metal stearate in
the polymerizable monomer composition, applying the bio-
compatible adhesive composite composition to living tissue
in need of treatment, and allowing the monomer in the bio-
compatible adhesive composite composition to polymerize
on the living tissue to form an adhesive composite material
comprising a polymer matrix comprising metal stearate
entrapped within a cyanoacrylate polymer matrix. The metal
stearate is present in an amount of at least 10% by weight of
the adhesive composite material.
BRIEF DESCRIPTION OF THE DRAWINGS
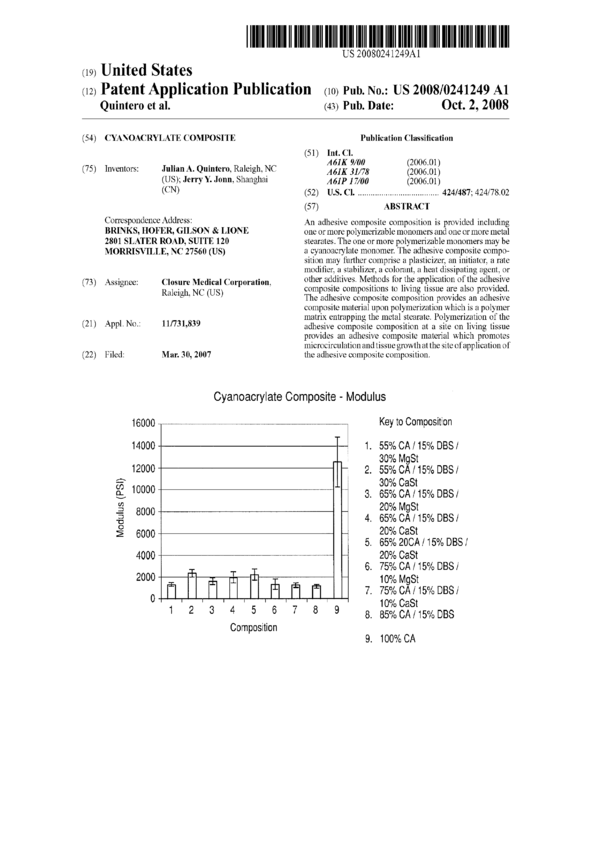
[0013] FIG. 1 is a graphical representation of the modulus
G’SI) of various adhesive composite materials as detailed i11
Example 1.
[0014] FIG. 2 is a graphical representation of the elongation
at break (inches) of various adhesive composite materials as
detailed in Example 1.
[0015] FIG. 3 is a graphical representation of the break
stress (PSI) of various adhesive composite materials as
detailed in Example 1.
DETAILED DESCRIPTION
[0016] An adhesive composite material is provided com-
prising a polymer matrix comprising one or more biocompat-
ible cyanoacrylate polymers and a plasticizer, and at least one
metal stearate entrapped in the polymer matrix. The at least
one metal stearate is present in an amount of at least 10% by
weight of the adhesive composite material. The adhesive
composite material is flexible and compliant, presenting a
distinguishable forn1 from cyanoacrylate adhesive materials
previously known which do not contain a metal stearate. The
adhesive composite material is a thickened, elastic, flexible,
bulky, and compliant polymer. The mechanical properties of
the adhesive composite material are comparable to those
obtained by the use of cyanoacrylate compositions without a
metal stearate, while providing advantages with regard to
viscosity and flexibility.
[0017] In other embodiments, absorbable cyanoacrylate
adhesive composite compositions may be prepared by com-
bining one or more metal stearates with polymerizable
cyanoacrylate monomer(s) whicl1 provide an absorbable
cyanoacrylate polymer upon polymerization. The combina-
tion of one or more absorbable polymerizable cyanoacrylate
monomers and one or more metal stearate results in an adhe-
sive composite composition or material with enhanced prop-
erties, such as controlled viscosity and setting time control in
the monomeric adhesive composite composition form, and
flexibility, rapid partial biodegradation and pore formation
once the adhesive composite composition undergoes poly-
US 2008/024l249 Al
merization to form a polymerized adhesive composite mate-
rial which provides a polymer matrix entrapping the metal
stearate.
[0018] When one or more metal stearates is combined with
one or more polymerizable monomers, the metal stearate and
polymerizable monomer or monomers form an adhesive
composite composition. “Adhesive composite composition”
as used herein refers to a combination of a metal stearate with
one or more polymerizable monomers or with a composition
comprising one or more polymerizable monomers. The
expressions “composition comprising one or more polymer-
izable monomers” and “polymerizable monomer composi-
ion” are used interchangeably and are used herein to refer to
a composition comprising one or more polymerizable mono-
ners which composition may also comprise one or more
additional components, such as initiator, plasticizer, inhibitor
or stabilizer, preservative, rate modifier, colorant, heat dissi-
oating agent, among others, which may be used in polymer-
izable monomer formulations. “Adhesive composite mate-
*ial” or “polymerized adhesive composite material” as used
ierein refers to the polymerized material or the polymer
natrix formed after polymerization of the polymerizable
nonoiner composition or the adhesive composite composi-
ion
[0019] The metal stearate and polymerizable monomer(s)
may be combined to form an adhesive composite composition
3y any means known to those of skill in the art, such as by
aringing the components into contact, mixing, blending, dis-
ributive mixing, dispersive mixing or other means.
[0020] In forming the adhesive composite composition,
when the metal stearate is combined with the polymerizable
nonoiner or monomers, a small amount of the metal stearate
oecomes partially dissolved while a substantial amount or
najority of the metal stearate becomes suspended in the poly-
nerizable monomer or polymerizable monomer composi-
ion. Thus, in embodiments, the adhesive composite compo-
sition is a suspension of metal stearate in polymerizable
nonoiner compos 'tion. “Suspension” as used herein refers to
a system in which metal stearate particles or particulates are
dispersed throughout a polymerizable monomer. In embodi-
nents, the metal s earate particulates are at least microscopi-
cally visible, and may be physically and chemically separated
from the polymerzable monomer composition in the adhe-
sive composite co nposition.
[0021] In embodiments, the metal stearate will form a sus-
pension when combined with a polymerizable cyanoacrylate
monomer compostion. Typically, a substantial portion of the
metal stearate is microscopically and physically distinguish-
able from the poly nerizable cyanoacrylate monomer compo-
sition in the adhesve composite composition thus formed. In
addition, upon polymerization of the polymerizable
cyanoacrylate monomer composition, a polymer matrix
forms in which the metal stearate is distinguishable from the
polymerized cyanoacrylate polymer matrix.
[0022] “Distinguishable” as used herein refers to the metal
stearate being differentiable as a substantially separate com-
ponent, e.g., a particulate component, within the suspension
with the polymerizable monomer composition or, upon poly-
merization, within the polymer matrix. The metal stearate
combined with one or more polymerizable monomers to form
a composite adhesive composition provides a viscosity
enhancing effect on the monomer or monomers or the mono-
mer composition, but remains a differentiable part of the
adhesive composite composition. Upon polymerization, the
Oct. 2, 2008
metal stearate in the adhesive composite material is substan-
tially entrapped in the polymer matrix formed from the poly-
merizable monomer or monomers.
[0023] Without being bound to any theory, it is believed that
the polymer matrix structure of the adhesive composite mate-
rial, when used in the body of a patient, allows for the metal
stearate to degrade or biodegrade within the polymer matrix
and/or allows for the metal stearate to diffuse through and/or
leach from the polymer matrix, forming a porous polymer
matrix. It is further believed that the metal stearate may
degrade or biodegrade orbe absorbed faster than the polymer
matrix can be absorbed in a patient’s body, or that the metal
stearate can diffuse through or leach from the polymer matrix
prior to the biodegradation or absorption of the polymer
matrix, forming a porous system. This porous polymermatrix
may provide a structure that allows microcirculation and tis-
sue growth through the porous polymer matrix. As used
herein, “degradation” refers to any manner of the metal stear-
ate exiting the polymer matrix which results in the formation
of a porous matrix. This egress of the metal stearate from the
polymer matrix is believed to form a porous matrix that pro-
motes microcirculation and tissue growth, therefore allowing
healing to take place.
[0024] The adhesive composite composition has enhanced
viscosity, thus avoiding previously known problems with the
use of polymerizable monomers. By way of example, one
problem with using monomeric cyanoacrylate compositions
in many medical applications is product run-off. This run-off
may cause the material to reach unintended locations. This is
a drawback in applications where precision is of importance,
particularly in medical applications where the cyanoacrylate
composition is applied in or on the body of a patient. The
adhesive composite composition and the polymerized adhe-
sive composite material of polymerizable cyanoacrylate
monomer(s) and metal stearate provides numerous advan-
tages, such as the elimination/reduction of run-off, precision,
elasticity, material memory, flexibility, bulkiness, and overall
good compliance to tissue. By way of example, an adhesive
composite composition comprising at least one cyanoacrylate
monomer and one or more metal stearates thus provides a
thickened material with enhanced viscosity that resists run-
off. The polymerized adhesive composite material provides
additional advantages, including, but not limited to, micro-
circulation and tissue growth through the porous structure of
the polymer matrix resulting from the degradation of the
metal stearate from the cyanoacrylate polymer matrix.
[0025] Another problem previously known in using poly-
merizable cyanoacrylate monomers to form cyanoacrylate
polymers was sometimes found in attaching tissue layers,
such as in seroma management. Polycyanoacrylate formed
from polymerizing cyanoacrylate monomer(s) may create a
physical barrier that separates tissue planes that need to be in
contact for appropriate healing. The adhesive composite
material comprising a polymer matrix of one or more bio-
compatible cyanoacrylate polymers and metal stearate
entrapped in the polymer matrix is believed to solve this
problem at least in part through rapid partial degradation,
biodegradation or diffusion of the metal stearate from the
adhesive composite material when used in or on the body of
a patient.
[0026] Previous attempts to solve the problems involved
with seroma management included the use of surgical drains.
The use of such drains increases cost, infection rates, and may
cause other complications. However, when an adhesive com-
US 2008/0241249 A1
posite composition of polymerizable cyanoacrylate mono-
mer(s) and one or more metal stearates is used to form a
cyanoacrylate polymer by polymerization of the one or more
cyanoacrylate monomers, the need for surgical drains may be
diminished as the dead space in the tissue may be eliminated
by the adherence of the tissue planes with the polymerized
cyanoacrylate composite material.
[0027] Suitable metal stearates for use in an adhesive com-
posite composition typically are substantially insoluble in the
polymerizable monomer or monomers, but may be readily
combined or mixed with the polymerizable monomer or
monomers. The metal stearates generally are used in the form
of freely flowable powders or particulates.
[0028] Suitable metal stearates include magnesium stear-
ate, aluminum stearate, calcium stearate, zinc stearate, or
mixtures thereof. In embodiments, the metal stearate may be
calcium stearate, aluminum stearate or magnesium stearate.
[0029] In embodiments, a metal stearate is selected which
is non-toxic or biocompatible and may be used in medical
applications. Particularly for medical uses, calcium stearate
may be used as the metal stearate.
[0030] The metal stearate may function in embodiments as
a viscosity enhancing agent. The increased viscosity, by way
of example, provides the ability to apply the adhesive com-
posite composition to a desired location without unwanted
“run-off” from the desired location.
[0031] In embodiments, a polymerizable cyanoacrylate
adhesive monomer composite composition will have an
effectively enhanced viscosity if it has a viscosity of about 10
to about 10,000 centipoise, preferably about 30 to about l ,500
centipoise, as measured with a Brookfield V iscometer at 25°
C. When the adhesive composite composition is to be used in
medical applications internally in a patient, the enhanced
viscosity preferably is about 100 to about 800 CP, as measured
with a Brookfield Viscometer at 25° C. When the adhesive
composite composition is to be used in medical applications
extemally on a patient, the enhanced viscosity preferably is
about 30 to about 100 cP, as measured with a Brookfield
Viscometer at 25° C.
[0032] The metal stearate in embodiments may be used in
an amount above about 10% of the total adhesive composite
composition and the polymerizable monomer composition
may be used in an amount from about 90% to about 65%. In
other embodiments, the metal stearate is used in an amount
from about 10 to about 25% of the total adhesive composite
composition and the polymerizable monomer composition is
present in an amount from about 90% to about 75%.
[0033] Adhesive composite compositions a11d adhesive
composite materials formed therefrom, are useful as tissue
adhesives, sealants for preventing bleeding or for covering
open wounds, implants for void space, and in other biomedi-
cal applications. The adhesive composite compositions and
the adhesive compo site materials resulting from polymeriza-
tion thereof find uses in, for example, preventing body fluid
leakage, sealing air leakage in the body, tissue approximation,
apposing surgically incised or traumatically lacerated tissues;
retarding blood flow from wounds; drug delivery; dressing
burns; dressing skin or other superficial or deep tissue surface
wounds (such as abrasions, chaffed or raw skin, and/or sto-
matitis); and aiding repair and regrowth of living tissue.
Adhesive composite compositions and adhesive composite
materials formed therefrom, have broad application for seal-
ing wounds in various living tissue, internal organs and blood
vessels, and can be applied, for example, 011 the interior or
Oct. 2, 2008
exteriorofbloodvessels and various organs or tissues. “Treat-
ing living tissue” as used herein refers to any of the above uses
or any other use wherein the adhesive composite composition
is applied on, to or into the body of a patient for either a
prophylactic or therapeutic purpose. ln embodiments, the
treatment of living tissue will be for a medical therapeutic
purpose.
[0034] Adhesive composite compositions, and polymers
formed therefrom, are also useful in industrial and home
applications, for example in bonding rubbers, plastics, wood,
composites, fabrics, and other natural and synthetic materials.
[0035] Suitable monomers are readily polymerizable, e.g.
anionically polymerizable or free radical polymerizable, or
polymerizable by zwitterions or ion pairs to form polymers.
Some such monomers are disclosed i11, for example, U.S. Pat.
No. 5,328,687 to Leung, et al., which is hereby incorporated
by reference herein in its entirety. Preferred monomers
include l,l-disubstituted ethylene monomers, such as a-cy-
anoacrylates. Preferably, the adhesive composite composi-
tions comprise one or more polymerizable cyanoacrylate
monomers and are biocompatible. The adhesive composite
compositions comprising one or more polymerizable
cyanoacrylate monomers may include combinations or mix-
tures of cyanoacrylate monomers.
[0036] The term “biocompatible” refers to a material being
suited for and meeting the requirements of a medical device,
used for either long or short term implants or for non-implant-
able applications, such that when implanted or applied in an
intended location, the material serves the intended function
for the required amount of time without causing an unaccept-
able response. Long term implants are defined as items
implanted for more than 180 days.
[0037] By way of example, useful monomers include a-cy-
anoacrylates of formula (I). These monomers are known in
the art and have the formula
(1)
CN
R2HC=C
COOR3
wherein R2 is hydrogen and R3 is a hydrocarbyl or substituted
hydrocarbyl group; a group having the formula —R4—O—
R5—O—R6, wherein R4 is a l,2-alkylene group having 2-4
carbon atoms, R5 is an alkylene group having 1-4 carbon
atoms, and R6 is an alkyl group having 1-6 carbon atoms; or
a group having the formula
—R7—c—o—R8
0
wherein R7 is
CH3
—(cH2),,—» —CH—» °I —c(cH3)2—»
wherein n is l-l0, preferably l-5 carbon atoms, and R8 is an
organic moiety.
US 2008/0241249 A1
[0038] Examples of suitable hydrocarbyl and substituted
hydrocarbyl groups include straight chain or branched chain
alkyl groups having 1-16 carbon atoms; straight chain or
branched chain C1-C16 alkyl groups substituted with an acy-
loxy group, a haloalkyl group, an alkoxy group, a halogen
atom, a cyano group, or a haloalkyl group; straight chain or
branched chain alkenyl groups having 2 to 16 carbon atoms;
straight chain or branched chain alkynyl groups having 2 to 12
carbon atoms; cycloalkyl groups; aralkyl groups; alkylaryl
groups; and aryl groups.
[0039] The organic moiety R3 may be substituted or unsub-
stituted and may be straight chain, branched or cyclic, satu-
rated, unsaturated or aromatic. Examples of such organic
moieties include C1-C8 alkyl moieties, C2-C8 alkenyl moi-
eties, C2-C8 alkynyl moieties, C3-C12 cycloaliphatic moi-
eties, aryl moieties such as phenyl and substituted phenyl and
aralkyl moieties such as benzyl, methylbenzyl, and phenyl-
ethyl. Other organic moieties include substituted hydrocar-
bon moieties, such as halo (e.g., chloro-, fluoro- and bromo-
substituted hydrocarbons) and oxy-substituted hydrocarbon
(e.g., alkoxy substituted hydrocarbons) moieties. Preferred
organic radicals are alkyl, alkenyl, and alkynyl moieties hav-
ing from 1 to about 8 carbon atoms, and halo-substituted
derivatives thereof. Particularly preferred are alkyl moieties
of 4 to 6 carbon atoms.
[0040] In the cyanoacrylate monomer of formula (I), R3
may be an alkyl group having 1-10 carbon atoms or a group
having the formula -AOR9, WhereinA is a divalent straight or
branched chain alkylene or oxyalkylene moiety having 2-8
carbon atoms, and R9 is a straight or branched alkyl moiety
having 1-8 carbon atoms.
[0041] Examples of groups represented by the formula
-AOR include 1-methoxy-2-propyl, 2-butoxy ethyl, isopro-
poxy ethyl, 2-methoxy ethyl, and 2-ethoxy ethyl.
[0042] The (x-cyanoacrylates of formula (I) canbe prepared
according to methods known in the art. U.S. Pat. Nos. 2,721,
858 and 3,254,111, each of which is hereby incorporated in its
entirety by reference, disclose methods for preparing ot-cy-
anoacrylates. For example, the 0.-cyanoacrylates can be pre-
pared by reacting an alkyl cyanoacetate with formaldehyde in
a nonaqueous organic solvent and in the presence of a basic
catalyst, followed by pyrolysis of the anhydrous intermediate
polymer in the presence of a polymerization inhibitor.
[0043] The CL-cyanoacrylates of formula (I) wherein R3 is a
group having the formula R44)—R3%)—R6 can be pre-
pared according to the method disclosed i11 U.S. Pat. No.
4,3 64,876 to Kimura et al., which is hereby incorporated in its
entirety by reference. In the Kimura et al. method, the (x-cy-
anoacrylates are prepared by producing a cyanoacetate by
esterifying cyanoacetic acid with an alcohol or by transesteri-
fying an alkyl cyanoacetate and an alcohol; condensing the
cyanoacetate and formaldehyde or para-formaldehyde in the
presence of a catalyst at a molar ratio of 0.5-1.5: 1, preferably
08-1221, to obtain a condensate; depolymerizing the con-
densation reaction mixture either directly or after removal of
the condensation catalyst to yield crude cyanoacrylate; and
distilling the crude cyanoacrylate to form a high purity
cyanoacrylate.
[0044] The CL-cyanoacrylates of formula (I) wherein R3 is a
group having the formula
Oct. 2, 2008
——R1—c——o——N
can be prepared according to the procedure described in U.S.
Pat. No. 3,995,641 to Kronenthal et al., which is hereby
incorporated in its entirety by reference. In the Kronenthal et
al. method, such or-cyanoacrylate monomers are prepared by
reacting an alkyl ester of an CL-cyanoacrylic acidwith a cyclic
1,3-diene to form a Diels-Alder adduct which is then sub-
jected to alkaline hydrolysis followed by acidification to form
the corresponding or-cyanoacrylic acid adduct. The (2-cy-
anoacrylic acid adduct is preferably esterified by an alkyl
bromoacetate to yield the corresponding carbalkoxymethyl
or-cyanoacrylate adduct. Altematively, the o.-cyanoacrylic
acid adduct may be converted to the or-cyanoacrylyl halide
adduct by reaction witl1 thionyl chloride. The or-cyanoacrylyl
halide adduct is then reacted with an alkyl hydroxyacetate or
a methyl substituted alkyl hydroxyacetate to yield the corre-
sponding carbalkoxymethyl or-cyanoacrylate adduct or car-
balkoxy alkyl or-cyanoacrylate adduct, respectively. The
cyclic 1,3-diene blocking group is finally removed and the
carbalkoxy methyl on-cyanoacrylate adduct or the carbalkoxy
alkyl or-cyanoacrylate adduct is converted into the corre-
sponding carbalkoxy alkyl or-cyanoacrylate by heating the
adduct in the presence of a slight deficit of maleic anhydride.
[0045] Examples of monomers of formula (I) include
cyanopentadienoates and or-cyanoacrylates of the formula:
(11)
CN
ZHC==C
COOR3
wherein Z is —CH:CH2 and R3 is as defined above. The
monomers of formula (II) wherein R3 is a11 alkyl group of
1-10 carbon atoms, i.e., the 2-cyanopenta-2,4-dienoic acid
esters, canbe prepared by reacting an appropriate 2-cyanoac-
etate with acrolein in the presence of a catalyst such as zinc
chloride. This method of preparing 2-cyanopenta-2,4-dienoic
acid esters is disclosed, for example, in U.S. Pat. No. 3,554,
990, which is hereby incorporated in its entirety by reference.
[0046] Suitable on-cyanoacrylate monomers which may be
used, alone or in combination, include alkyl oi-cyanoacrylates
such as 2-octyl cyanoacrylate; dodecyl cyanoacrylate; 2-eth-
ylhexyl cyanoacrylate; butyl cyanoacrylate such as 11-butyl
cyanoacrylate; ethyl cyanoacrylate; methyl cyanoacrylate or
other 0.-cyanoacrylate monomers such as methoxyethyl
cyanoacrylate; 2-ethoxyethyl cyanoacrylate; 3-methoxybutyl
cyanoacrylate; 2-butoxyethyl cyanoacrylate; 2-isopropoxy-
ethyl cyanoacrylate; and 1-methoxy-2-propyl cyanoacrylate.
In embodiments, the monomers are ethyl, n-butyl, or 2-octyl
or-cyanoacrylate.
[0047] Other cyanoacrylates which may be used include
alkyl ester cyanoacrylates. Besides the process detailed
above, alkyl ester cyanoacrylates can also be prepared
through the Knoevenagel reaction of an alkyl cyanoacetate, or
an alkyl ester cyanoacetate, with paraformaldehyde. This
leads to a cyanoacrylate oligomer. Subsequent thermal crack-
ing of the oligomer results in the formation of a cyanoacrylate
US 2008/0241249 A1
monomer. After further distillation, a cyanoacrylate mono-
mer with high purity (greater than 95.0%, preferably greater
than 99.0%, and more preferably greater than 99.8%), may be
obtained.
[0048] Monomers prepared with low moisture content and
essentially free of impurities (e.g., surgical grade) are pre-
ferred for biomedical use. Monomers utilized for industrial
purposes need not be as pure.
[0049] In some embodiments, the alkyl ester cyanoacrylate
monomers may have the formula:
0 R1’ R2’
NC 0
CH; 0
wherein R1’ a11d R2’ are, independently, H, a straight,
branched or cyclic alkyl, or are combined together in a cyclic
alkyl group, R3’ is a straight, branched or cyclic alkyl group,
andm is 1-8. Preferably, R1’ is H ora C1, C3 or C3 alkyl group,
such as methyl or ethyl; R2‘ is H or a C 1, C2 or C3 alkyl group,
such as methyl or ethyl; R3’ is a Cl-C16 alkyl group, more
preferably a C1-C10 alkyl group, such as methyl, ethyl, pro-
pyl, isopropyl, butyl, isobutyl, pentyl, hexyl, heptyl, octyl,
nonyl or decyl, and even more preferably a C3, C3 or C4 alkyl
group, and m is preferably l-4.
[0050] Examples of the alkyl ester monomers may include,
but are not limited to:
CN
H O O or
W27!“ T] V
H O O
3—(2—Cyano—acryloyloxy)—butyric acid ethyl ester
(Et—[$-HBT-CA)
CN
“W0 O“
H O O
3-(:2—Cyano-acryloyloxy)-hexanoic acid ethyl ester
(Et-|3—CPL-CA)
[0051] Additional examples of alkyl ester cyanoacrylates
include, but are not limited to, butyl lactoyl cyanoacrylate
(BLCA), butyl glycoloyl cyanoacrylate (BGCA), isopropyl
glycoloyl cyanoacrylate (IPGCA), ethyl lactoyl cyanoacry-
late GELCA), and ethyl glycoloyl cyanoacrylate (EGCA) and
combinations thereof. BLCA may be represented by the for-
mula above, wherein R1’ is H, R2’ is methyl and R3’ is butyl.
BGCA may be represented by the formula above, wherein R1‘
is H, R2’ is H and R3 V is butyl. lPGCA may be represented by
the formula above, wherein R1’ is H, R2’ is H and R3’ is
isopropyl. ELCA may be represented by the formula above,
wherein R1’ is H, R2’ is methyl and R3 is ethyl. EGCA may be
represented by the formula above, wherein R1’ is H, R2‘ is H
and R3 V is ethyl.
Oct. 2, 2008
[0052] Other examples of alkyl ester cyanoacrylates
include alkyl alpha-cyanoacryloyl caprolactate and alkyl
alpha-cyanoacryloyl butrylactate. Other cyanoacrylates use-
ful in the present invention are disclosed in U.S. Pat. No.
3,995,641 to Kronenthal et al., the entire disclosure of which
is hereby incorporated by reference.
[0053] Altematively, or in addition, alkyl ether cyanoacry-
late monomers may be used. Alkyl ethyl cyanoacrylates have
the general formula:
NC R” R”
wherein R1” is a straight, branched or cyclic alkyl, and R2” is
a straight, branched or cyclic alkyl group. Preferably, R1" is a
C1, C2 or C3 alkyl group, such as methyl or ethyl; and R2” is a
C1-C16 alliyl group, more preferably a C1-C10 alkyl group,
such as methyl, ethyl, propyl, isopropyl, butyl, isobutyl, pen-
tyl, hexyl, heptyl, octyl, nonyl or decyl, and even more pref-
erably a C2, C3 or C4 alkyl group.
[0054] Examples of alkyl ether cyanoacrylates include, but
are not limited to, isopropyoxy ethyl cyanoacrylate (IPECA)
and methoxy butyl cyanoacrylate (MBCA) or combinations
thereof. IPECA may be represented by the formula above,
wherein R1” is ethylene and R2” is isopropyl. MBCA may be
represented by the formula above, wherein R1” is n-butylene
and R2" is methyl.
[0055] Alkyl ester cyanoacrylates and alkyl ether
cyanoacrylates are particularly useful for medical applica-
tions because of their absorbability by living tissue and asso-
ciated fluids. The terms “absorbable” or “absorbable adhe-
sive” or variations thereof mean the ability of a tissue-
compatible material to degrade or biodegrade at some time
after implantation into products that are eliminated from the
body or metabolized therein. Thus, as used herein, absorb-
ability means that the polymerized adhesive is capable of
being absorbed, either fully or partially, by tissue after appli-
cation of the adhesive.
[0056] Likewise, the terms “non-absorbable” or “non-ab-
sorbable adhesive” or variations thereof mean completely or
substantially incapable of being absorbed, either fully or par-
tially, by tissue after application of the adhesive. Furthermore,
relative terms such as “faster absorbing” and “slower absorb-
ing” are used relative to two monomer species to indicate that
a polymer produced from one monomer species is absorbed
faster (or slower) than a polymer formed from the other
monomer species.
[0057] For the purposes herein, the term “substantially
absorbed” means at least 90% absorbed within about three
years. Likewise, the term “substantially non-absorbed”
means at most 20% absorbed within about three years. Pref-
erably, l00% of the polymerized and applied cyanoacrylate
when using these types of cyanoacrylate monomers may be
absorbed in a period of less than 3 years, preferably approxi-
mately 2-24 months, more preferably 3-18 months, and most
preferably 6-12 months after application of the adhesive to
living tissue. The absorption time may vary depending on the
particular uses and tissues involved. Thus, for example,
longer absorption time may be desired where the adhesive
composition is applied to hard tissues, such as bone, but a
US 2008/0241249 A1
faster absorption time may be desired where the adhesive
composite composition is applied to softer tissues.
[0058] The selection of monomer will affect the absorption
rate of the resultant polymer, as well as the polymerization
rate of the monomer. Two or more different monomers that
have varied absorption and/or polymerization rates may be
used in combination to give a greater degree of control over
the absorption rate of the resultant polymer, as well as the
polymerization rate of the monomer.
[0059] According to some embodiments, the adhesive
composite composition comprises a mixture of monomer
species with varying absorption rates. Where two monomer
species having different absorption rates are used, it is pre-
ferred that the absorption rates be sufiiciently different that a
mixture of the two monomers can yield a third absorption rate
that is effectively different from the absorption rates of the
two monomers individually. Compositions according to these
embodiments are described, for example, in U.S. patent
application Ser. No. 09/919,877, filed Aug. 2, 2001, pub-
lished as U.S. Patent Publication No. 2002/0037310 on Mar.
28, 2002, and U.S. Pat. No. 6,620,846, both incorporated
herein by reference in their entireties.
[0060] Absorbable cyanoacrylates have broad application
for closure and hemostatic sealing of wounds and the like in
various livi11g tissue, including but not limited to internal
organs and blood Vessels. These absorbable formulations can
be applied on the interior or exterior of various organs and
tissues.
[0061] Adhesive composite compositions as disclosed
preferably are biocompatible and may be applied internally or
extemally in or on living tissue. The adhesive composite
compositions are preferably sterilized for use in medical
applications. More preferably, the adhesive composite com-
positions may be sterilized by dry heat sterilization while
retaining suitability for medical applications.
[0062] For example, suitable adhesive composite composi-
tions according to embodiments can be prepared by mixing
suitable quantities of an alkyl alpha cyanoacrylate such as
2-octyl alpha-cyanoacrylate with one of butyl lactoyl
cyanoacrylate (BLCA), butyl glycoloyl cyanoacrylate
(BGCA), isopropyl g ycoloyl cyanoacrylate (IPGCA), ethyl
lactoyl cyanoacryla e GELCA), and ethyl glycoloyl
cyanoacrylate (EGCA). Such mixtures may range from ratios
of about 90:10 to about 10:90 by weight, preferably about
75:25 to about 25:75 3y weight such as from about 60:40 to
about 40:60 by weight.
[0063] In embodiments, the metal stearate and the polymer-
izable monomer comoosition are not combined to form the
adhesive composite composition until just prior to or at the
time of use. Thus, the metal stearate may comprise a first
component and the polymerizable monomer composition
may comprise a seco id component in a system for treating
livi11g tissue. A two component system may be used, by way
of example, where the metal stearate effectively initiates or
accelerates the polymerization of the polymerizable mono-
mer composition. Besides polymerizable monomer(s), the
polymerizable monomer composition may comprise one or
more additional constituents.
[0064] By way of example, stabilizing agents may be used
in the polymerizable monomer composition. Suitable free
radical stabilizing agents foruse in polymerizable cyanoacry-
late adhesive composite compositions comprising one or
more polymerizable cyanoacrylate monomers include hydro-
quinone, hydroquinone monomethyl ether, catechol, pyro-
Oct. 2, 2008
gallol, benzoquinone, 2-hydroxybenzoquinone, p-methoxy
phenol, t-butyl catechol, butylated hydroxy ani sole, butylated
hydroxy toluene, and t-butyl hydroquinone and mixtures or
combinations thereof. The free radical stabilizing agents may
be used in amounts from about 5 to about 10,000 ppm. In
embodiments, if hydroquinone is used, the amount may be
from about 5 to about 70 ppm and may be used in conjunction
with butylated hydroxy anisole in an amount of about 500 to
about 10,000 ppm.
[0065] Cyanoacrylate adhesive composite compositions
comprising one or more polymerizable cyanoacrylate mono-
mers may also optionally include both at least one anionic
vapor phase stabilizer and at least one anionic liquid phase
stabilizer. These stabilizing agents inhibit polymerization.
Examples of such anionic agents are described for example,
in U.S. Pat. No. 6,620,846, incorporated herein by reference
in its entirety.
[0066] The anionic vapor phase stabilizers may be selected
from among known stabilizers, including, but not limited to,
sulfur dioxide or hydrogen fluoride. The amount of anionic
vapor phase stabilizer that is added to the monomer compo-
sition depends on the identity of the liquid phase stabilizer(s)
chosen in combination with it, the monomer to be stabilized,
as well as the packaging material to be used for the compo-
sition. Typically, each anionic vapor phase stabilizer is added
to give a concentrationof less than about 200parts per million
(ppm). In embodiments, each anionic vapor phase stabilizer is
present in an amount from about 1 to about 200 ppm, prefer-
ably from about 10 to about 75 ppm, even more preferably
from about 10 to about 50 ppm, and most preferably from
about 10 to about 20 ppm. The amount to be used can be
determined by one of ordinary skill in the art using known
techniques without undue experimentation.
[0067] In embodiments, the liquid phase anionic stabilizer
is a very strong acid. As used herein, a very strong acid is an
acid that has an aqueous pK,, of less than 1.0. Suitable very
strong acidic stabilizing agents include, but are not limited to,
very strong mineral and/or oxygenated acids. Examples of
such very strong acids include, but are not limited to, sulfuric
acid (pK,,—3 .0), perchloric acid (pKa—5), hydrochloric acid
(pK,,—7.0), hydrobromic acid (pK,,—9), fluorosulfonic acid
(pK,,