Viscous Alpha-Cyanoacrylate Compositions
Folder:
Year:
Abstract:
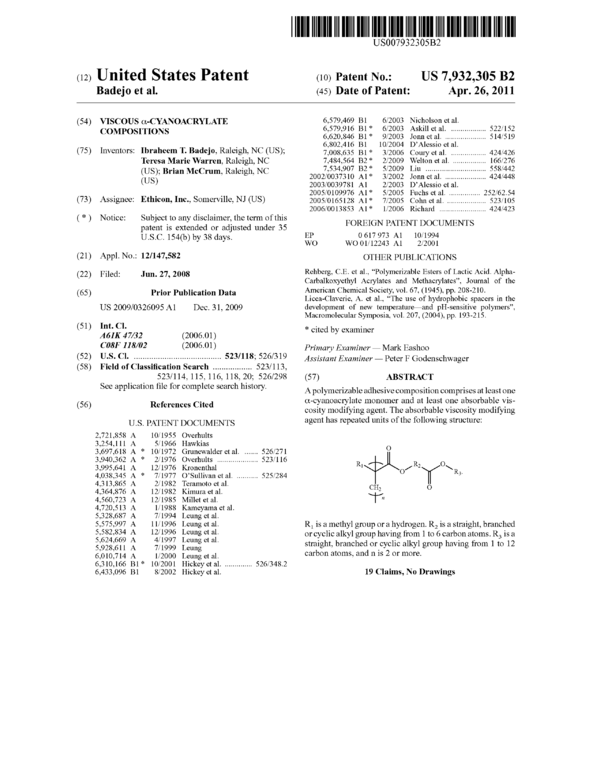
A polymerizable adhesive composition comprises at least one α-cyanoacrylate monomer and at least one absorbable viscosity modifying agent. The absorbable viscosity modifying agent has repeated units of the following structure: - R1 is a methyl group or a hydrogen. R2 is a straight, branched or cyclic alkyl group having from 1 to 6 carbon atoms. R3 is a s straight, branched or cyclic alkyl group having from 1 to 12 carbon atoms, and n is 2 or more.
Type of document:
Language:
US007932305B2
(12) Ulllted States Patent (10) Patent No.: US 7,932,305 B2
Badejo et al. (45) Date of Patent: Apr. 26, 2011
94>
, , s 1 eta . ................ ..
COMPOSITIONS 6,620,846 B144 9/2003 Jonn et a1. ................... H 514/519
_ 4 6,802,416 B1 10/2004 D’Alessio et al.
(75) IHVGIHOFSI Ibraheem T-3391610: Ra1e1ghsNC(US); 7,008,635 B1* 3/2006 Coury et al. ................ .. 424/426
Teresa Marie Warren, Raleigh, NC 7,484,564 B2 * 2/2009 Welton et al. .. 166/276
. - ' 7,534,907 B2* 5/2009 Liu ....... .. 558/442
(33). Bnan Mccrum. Ra1e1gh.NC 200,000,040 44 .,. 0000, 0000 00 ,0 0000000000000000000 0 424,440
( ) 2003/0039781 A1 2/2003 D’Alessio et al.
0 _ 0 2005/0109976 A1* 5/2005 Fuchs et al. .............. .. 252/62.54
(73) Asslgneet Eth1c0n,Inc-, S0merV111e,NJ (US) 2005/0165128 A1* 7/2005 Cohn etal. .. 523/105
2006/0013853 A1* 1/2006 R1 h d ...................... .. 424/423
( * ) Notice: Subject to any disclaimer, the term of this FOREIGN PATENTCDZCUMENTS
patent is extended or adjusted under 35
154bv38 00:22:: :1 12/122:
(21) App1.N0.: 12/147,582 OTHER PUBLICATIONS
(22) Filed: Jun_ 27, 2008 Rehberg, C.E. et al., “Polymerizable Esters of Lactic Acid. Alpha-
Carbalkoxyethyl Acrylates and Methacrylates”, Journal of the
(65) Prior Publication Data American Chemical Society, vol. 67, (1945), pp. 208-210. _
Licea-Claverie, A. et al., “The use of hydrophobic spacers in the
US 2009/0326095 A1 Dec, 31, 2009 development of new temperature—and pH-sensitive polymers”,
Macromolecular Syrnposia, vol. 207, (2004), pp. 193-215.
A61K 47/32 (2006.01) we y exammer
C08F 118/02 (200601) Primary Examiner — Mark Eashoo
(52) U.S. Cl. ...................................... .. 523/118; 526/319 Assistant Examiner _ peter F Godenschwager
(58) Field of Classification Search ................ .. 523/113,
523/114, 115, 116, 118, 20, 526/298 (57) ABSTRACT
See application file for Complete Search history’ A polymerizable adhesive composition comprises at least one
(56) References Cited 01-cyanoacrylate monomer and at least one absorbable vis-
cosity modifying agent. The absorbable viscosity modifying
US. PATENT DOCUMENTS agent has repeated units of the following structure.
2,721,858 A 10/1955 Overhults
3,254,111 A 5/1966 Hawkias
3,697,618 A * 10/1972 Grunewalder et al. ..... .. 526/271 0
3,940,362 A * 2/1976 Overhults ................... .. 523/116 R R 0
3,995,641 A 12/1976 Kronenthal 1 0/ 2 \R
4,038,345 A * 7/1977 O’Sullivan et al. ......... .. 525/284 \n/ 3'
4,313,865 A 2/1982 Teramoto et al. CH 0
4,364,876 A 12/1982 Kimura et al. 2
4,560,723 A 12/1985 Millet et al. \l/”
4,720,513 A 1/1988 Kameyama et al.
5,328,687 A 7/1994 Leung et al.
5,575,997 A 11/1996 Leung et a1. R1 is a methyl group or a hydrogen. R2 is a straight, branched
5582334 A 12/1996 Leung 91 31- or cyclic alkyl group having from 1 to 6 carbon atoms. R3 is a
5’624’669 A 4/1997 Leung et 31' strai ht branched or c clic alkyl rou havin from 1 to 12
5,928,611 A 7/1999 Leung g ’ . y g P g
6,010,714 A 1/2000 Leung et 31. carbon atoms, and n is 2 or more.
6,310,166 B1* 10/2001 Hickey et al. ............ .. 526/348.2
6,433,096 B1 8/2002 Hickey et al. 19 Claims, N0 Drawings
US 7,932,305 B2
1
VISCOUS (X-CYANOACRYLATE
COMPOSITIONS
FIELD OF THE INVENTION
The invention relates to absorbable viscosity modifying
agents for adhesives or sealants, and more particularly to
cyanoacrylate monomer compositions. This invention further
relates to the use of such viscosity modifying agents and
cyanoacrylate monomers as tissue adhesives/ sealant compo-
sitions in surgical, medical and industrial applications, and to
the production thereof.
BACKGROUND
Monomer and polymer adhesives/ sealants are used in both
industrial (including household) and medical/ surgical appli-
cations. Included among these adhesives or sealants are
cyanoacrylates monomers and polymers resulting therefrom.
Since the discovery of the adhesive/ sealant properties of such
monomers and polymers, they have found wide use due to the
speed with which they cure, the strength of the resulting bond
formed, and their relative ease of use. These characteristics
have made ot-cyanoacrylate compositions the primary choice
for numerous adhesive applications such as bonding plastics,
rubbers, glass, metals, wood, and, more recently, medical,
biological or living tissues.
Medical and surgical applications of ot-cyanoacrylate com-
positions include their use as altemates or adjuncts to surgical
sutures, meshes and staples or other medical devices in
wound closure, as well as for covering and protecting surface
wounds such as lacerations, abrasions, burns, stomatitis,
sores, and other surface wounds. When an ot-cyanoacrylate
composition is applied, it is usually applied in its monomeric
form, and the resultant polymer creates the desired adhesive
bond or sealant strength.
At standard temperatures, the monomeric ot-cyanoacrylate
may run when applied to surfaces. As a result, the 0t-cy-
anoacrylate adhesive may spread into a wound or along a
surface to which it has been applied to areas that do not
require an adhesive and that may be adversely affected by an
adhesive. Therefore, it is desirable to control the viscosity of
the monomeric ot-cyanoacrylate composition in order to pre-
vent escape of the adhesive from the area of application. In
order to reduce run-off or achieve a suitably viscous adhesive,
a thickening or viscosity modifying agent may be added to a
monomeric ot-cyanoacrylate composition.
Polymerizable ot-cyanoacrylate monomers and composi-
tions comprising such monomers are disclosed in U.S. Pat.
No. 5,328,687. Further, the use of polymers as thickening
agents for ot-cyanoacrylate monomers and compositions is
disclosed in U.S. Pat. No. 6,433,096. Each ofthe above docu-
ments is incorporated by reference herein in its entirety.
For some medical applications, an absorbable thickening
or viscosity modifying agent provides benefits over a non-
absorbable thickening or viscosity modifying agent. For
example, it is desirable to have a monomer-based internal
adhesive or sealant composition that polymerizes in vivo,
where the monomer, the composition thereof, and the result-
ant polymer are biocompatible. It is also desirable to use an
adhesive or sealant composition that fills intemal cavities and
voids, penetrating and conforming to the interstices and pores
of the tissue, prior to curing or setting. Finally, it is desirable
that the resultant polymer also be biodegradable, so that the
degradation products are completely eliminated from the
human body as waste products.
10
15
20
25
30
35
40
45
50
55
60
65
2
Therefore, there is a need for an absorbable and biocom-
patible viscosity modifying agent for ot-cyanoacrylate com-
positions that exhibits controlled viscosity and absorbability
sufficient for medical applications and produces a polymer
adhesive/ sealant that may also be biodegradable.
While it is desirable to have an adhesive that is biodegrad-
able, achieving such biodegradability should not adversely
affect other desirable properties of the adhesive, e.g., stability.
In order for an adhesive to be a commercially viable product,
it is preferred that the monomeric form of the adhesive have a
shelf life of at least one year at standard, non-refrigerated,
temperatures.
Therefore, there is a need for an ot-cyanoacrylate adhesive
composition that is absorbable and biocompatible and also
stable enough to be commercially viable.
SUMMARY
The present invention relates to a monomer composition
comprising at least one ot-cyanoacrylate monomer and at
least one absorbable viscosity modifying agent, which mono-
mer polymerizes to form an adhesive or sealant possessing
controlled viscosity and is minimally toxic to non-toxic.
The absorbable viscosity modifying agent has repeated
units of the following structure:
R1
0
R2 0
0/ T \ R3
0
CH2
‘1’»«
wherein R1 is a methyl group or a hydrogen, R2 is a straight,
branched or cyclic alkyl group having from 1 to 6 carbon
atoms, R3 is a straight, branched or cyclic alkyl group having
from 1 to 12 carbon atoms, and n is 2 or more.
The present invention includes many aspects and features.
The at least one absorbable viscosity modifying agent may
be selected from the group consisting of a polymer of 2-meth-
oxy-2-oxoethyl acrylate, 2-ethoxy-2-oxoethyl acrylate,
2-oxo-2-propoxyethyl acrylate, 2-butoxy-2-oxoethyl acry-
late, 2-methoxy-2-oxoethyl methacrylate, 2-ethoxy-2-oxoet-
hyl methacrylate, 2-oxo-2-propoxyethyl methacrylate, 2-bu-
toxy-2-oxoethyl methacrylate, l-methoxy-l -oxopropan-2-yl
acrylate, l-ethoxy- l -oxopropan-2 -yl acrylate, l-oxo- 1 -pro-
poxypropan-2-yl acrylate, l-butoxy-l-oxopropan-2-yl acry-
late, 1 -methoxy- l -oxopropan-2 -yl methacrylate, l-ethoxy-l -
oxopropan-2 -yl methacrylate, l -oxo- l -propoxypropan-2-yl
methacrylate, l -butoxy- l -oxopropan-2 -yl methacrylate, and
mixtures thereof.
DETAILED DESCRIPTION
For the purposes of this invention, the term “absorbable” or
variations thereof means capable of being absorbed, degraded
or biodegraded, either fully or partially, by animal (including
human) tissue after application of the adhesive or sealant.
Also, the term “substantially absorbed” means at least 90%
absorbed. The term “non-absorbable” or variations thereof
means completely or substantially incapable of being
absorbed, either fully or partially, by animal tissue after appli-
cation of the adhesive or sealant.
The term “effective amount” is an amount sufficient to
provide desired properties to the adhesive compositions. The
US 7,932,305 B2
3
effective amount may be affected by ot-cyanoacrylate mono-
mers, viscosity modifying agents, stabilizers, initiators or
other ingredients used to form the adhesive composition.
The term “stability” or “stabilized” as used herein may be
determined by measuring the viscosity of the 0t-cyanoacry-
late composition over a period of time. Premature polymer-
ization of the ot-cyanoacrylate composition results in an
increase in viscosity over time; therefore, viscosity of a com-
position may be used to determine composition stability.
The adhesive/ sealant compositions of the present invention
comprise a new member of carbalkoxyalkyl acrylate or car-
balkoxyalkyl methacrylate as an absorbable viscosity modi-
fying agent. The new members of carbalkoxyalkyl acrylate or
carbalkoxyalkyl methacrylate have repeated units of the fol-
lowing structure;
0
R1 R2 0
\‘J9kO/ \”/ \R3
0
CH2
‘W
wherein R1 is a methyl group or a hydrogen, R2 is a straight,
branched or cyclic alkyl group having from 1 to 6 carbon
atoms, R3 is a straight, branched or cyclic alkyl group having
from 1 to 12 carbon atoms, and n is 2 or more.
An exemplary new member of carbalkoxyalkyl methacry-
late is 1-butoxy-1-oxopropan-2-yl methacrylate or butyl lac-
toyl methacrylate (BLMA). The BLMA monomer and poly-
mer are not available commercially, and may be synthesized
as described herein in Example 1. The BLMA polymer is
particularly useful as a viscosity modifying agent because it is
compatible with ot-cyanoacrylate monomers. Also, the
BLMA polymer is preferred for certain medical applications
as a viscosity modifying agent because is absorbable. The
addition of an absorbable BLMA polymer to an absorbable
adhesive composition provides controlled viscosity and
maintains the absorbability of the adhesive composition.
Thus the resulting adhesive polymer formed thereof may be
absorbable or substantially absorbed by living tissues. The
BLMA polymer has repeated units of
O
OJ\’(O\/\/
O
In embodiments, the BLMA polymer has a high molecular
weight, preferably at least 250,000 Daltons and more prefer-
ably at least 500,000 Daltons. The BLMA polymer is soluble
in an ot-cyanoacrylate monomer composition at room tem-
perature (i.e., 20-25° C.). Thus it may be added to the mono-
mer composition without excessive heating of the composi-
tion and may remain uniformly combined in the composition.
The BLMA polymer may be used in ot-cyanoacrylate adhe-
sive compositions in an effective amount, for example, from
about 1.0% to about 15.0% by weight of the adhesive com-
position. The BLMA polymer may be used in an amount from
about 2.0% to about 8.0% by weight, preferably, from about
5.0% to about 7.5% by weight of the adhesive composition.
H3C
CH2
‘W
10
15
20
25
30
35
40
45
50
55
60
65
4
The effective amount of the BLMA polymer in the adhesive
composition may provide a viscosity of about 20 to about
1,000 centipoise, preferably about 50 to about 800 centipoise,
as measured with a Brookfield Viscometer at 25° C.
Other suitable members of carbalkoxyalkyl acrylate or car-
balkoxyalkyl methacrylate, new or known include, but are not
limited to, 2-methoxy-2-oxoethyl acrylate, 2-ethoxy-2-oxo-
ethyl acrylate, 2-oxo-2-propoxyethyl acrylate, 2-butoxy-2-
oxoethyl acrylate, 2-methoxy-2-oxoethyl methacrylate,
2-ethoxy-2-oxoethyl methacrylate, 2-oxo-2-propoxyethyl
methacrylate, 2-butoxy-2-oxoethyl methacrylate, 1-meth-
oxy-1-oxopropan-2-yl acrylate, 1-ethoxy-1-oxopropan-2-yl
acrylate, 1-oxo-1-propoxypropan-2-yl acrylate, 1-butoxy-1-
oxopropan-2-yl acrylate, 1-methoxy-1-oxopropan-2-yl
methacrylate, 1 -ethoxy- 1 -oxopropan-2-yl methacrylate, and
1 -oxo-1-propoxypropan-2-yl methacrylate. The polymers of
above listed members of carbalkoxyalkyl acrylate or car-
balkoxyalkyl methacrylate, either alone or in combination,
may be used as viscosity modifying agents herein.
The sealant/adhesive composition of the present invention
also comprises at least one polymerizable ot-cyanoacrylate
monomer. Preferably, the adhesive composition comprises
one or more polymerizable ot-cyanoacrylate monomers and
may include combinations or mixtures of ot-cyanoacrylate
monomers.
The 01-cyanoacrylates monomers are known in the art and
have the formula (I)
(1)
CN
R2HC=C
COOR3
wherein R2 is hydrogen and R3 is a hydrocarbyl or substituted
hydrocarbyl group; a group having the formula —R4—O—
R5—O—R6, wherein R4 is a 1,2-alkylene group having 2-4
carbon atoms, R5 is an alkylene group having 1-4 carbon
atoms, and R6 is an alkyl group having 1-6 carbon atoms; or
a group having the formula
—R7—C—o—R8
0
wherein R7 is
CH3
—(CH2),.—, —CH—, or —C(CH2)2—,
wherein n is 1-10, preferably 1-5 carbon atoms, and R8 is an
organic moiety. The organic moiety R8 may be substituted or
unsubstituted and may be straight chain, branched or cyclic,
saturated, unsaturated or aromatic. Preferred organic radicals
are alkyl, alkenyl, and alkynyl moieties having from 1 to
about 8 carbon atoms, and halo-substituted derivatives
thereof. Particularly preferred are alkyl moieties of 4 to 6
carbon atoms.
In the ot-cyanoacrylate monomer of formula (I), R3 may be
an alkyl group having 1-10 carbon atoms or a group having
the formula -AOR9, wherein A is a divalent straight or
branched chain alkylene or oxyalkylene moiety having 2-8
carbon atoms, and R9 is a straight or branched alkyl moiety
US 7,932,305 B2
5
having 1-8 carbon atoms. Examples of groups represented by
the formula -AOR9 include 1-methoxy-2-propyl, 2-butoxy
ethyl, isopropoxy ethyl, 2-methoxy ethyl, and 2-ethoxy ethyl.
The 01-cyanoacrylates of formula (I) can be prepared
according to methods known in the art. For example, 0t-cy-
anoacrylates can be prepared by reacting an alkyl cyanoac-
etate with formaldehyde in a nonaqueous organic solvent and
in the presence of a basic catalyst, followed by pyrolysis of
the anhydrous intermediate polymer in the presence of a
polymerization inhibitor as disclosed in U.S. Pat. Nos. 2,721,
858 and 3,254,111. The 01-cyanoacrylates of formula (I)
wherein R1 is a group having the formula R4—O—R3—O—
R6 can be prepared according to the method disclosed in U.S.
Pat. No. 4,364,876, and the ot-cyanoacrylates of formula (I)
wherein R3 is a group having the formula
—R7—C—o—R8
O
can be prepared according to the method described in U.S.
Pat. No. 3,995,641. Each of the above listed patents is hereby
incorporated by reference in its entirety.
Suitable ot-cyanoacrylate monomers may be used, alone or
in combination, and may include, but not be limited to, 2-oc-
tyl cyanoacrylate; dodecyl cyanoacrylate; 2-ethylhexyl
cyanoacrylate; butyl cyanoacrylate such asn-butyl
cyanoacrylate; ethyl cyanoacrylate; methyl cyanoacrylate;
methoxyethyl cyanoacrylate; 2-ethoxyethyl cyanoacrylate;
3-methoxybutyl cyanoacrylate; 2-butoxyethyl cyanoacry-
late; 2-isopropoxyethyl cyanoacrylate; and 1-methoxy-2-
propyl cyanoacrylate. In embodiments, the monomers may
be ethyl, n-butyl, or 2-octyl ot-cyanoacrylate.
The ot-cyanoacrylate monomers which may be used in the
adhesive/sealant compositions may include alkyl ester
cyanoacrylates. The alkyl ester cyanoacrylate monomers may
have the formula:
NC 0
CH2 0
wherein R1" and R2" are, independently, H, a straight,
branched or cyclic alkyl, or are combined together in a cyclic
alkyl group, R3 " is a straight, branched or cyclic alkyl group,
andm is 1-8. Preferably, R1" is H ora C1, C2 or C3 alkyl group,
such as methyl or ethyl; R2 is H or a C1, C2 or C3 alkyl group,
such as methyl or ethyl; R3" is a C1-C16 alkyl group, more
preferably a C1-C10 alkyl group, such as methyl, ethyl, pro-
pyl, isopropyl, butyl, isobutyl, pentyl, hexyl, heptyl, octyl,
nonyl or decyl, and even more preferably a C2, C3 or C4 alkyl
group, and m is preferably 1-4.
Examples of alkyl ester cyanoacrylates include, but are not
limited to, butyl lactoyl cyanoacrylate (BLCA), butyl gly-
coloyl cyanoacrylate (BGCA), isopropyl glycoloyl
cyanoacrylate (IPGCA), ethyl lactcyl cyanoacrylate (ELCA),
and ethyl glycoloyl cyanoacrylate (EGCA) and combinations
thereof. BLCA may be represented by the formula above,
wherein R1" is H, R2" is methyl and R3 " is butyl. BGCA may be
represented by the formula above, wherein R1" is H, R2" is H
and R3 is butyl. IPGCA may be represented by the formula
above, wherein R1" is H, R2" is H and R3 " is isopropyl. ELCA
10
15
20
25
30
35
40
45
50
55
60
65
6
may be represented by the formula above, wherein R1" is H,
R2" is methyl and R3 " is ethyl. EGCA may be represented by
the formula above, wherein R1" is H, R2" is H and R3 " is ethyl.
Other examples of alkyl ester cyanoacrylates include 3-(2-
Cyano-acryloyloxybutyric acid ethyl ester (Et-[3-HBT-CA),
3-(2-cyano-acryloyloxy)-hexanoic acid ethyl ester (Et-[3-
CPL-CA), alkyl alpha-cyanoacryloyl caprolactate and alkyl
alpha-cyanoacryloyl butrylactate.
The alkyl ester ot-cyanoacrylate monomers may be pre-
pared through the Knoevenagel reaction of an alkyl cyanoac-
etate, or an alkyl ester cyanoacetate, with paraformaldehyde
as disclosed in U.S. Pat. No. 3,995,641. This leads to a
cyanoacrylate oligomer. Subsequent thermal cracking of the
oligomer results in the formation of a ot-cyanoacrylate mono-
mer. After further distillation, an ot-cyanoacrylate monomer
with high purity (greater than 95.0%, preferably greater than
99.0%, and more preferably greater than 99.8%) may be
obtained. Monomers prepared with low moisture content and
essentially free of impurities (e.g., surgical grade) are pre-
ferred for biomedical use.
An alternative or additional ot-cyanoacrylate which may be
used in the adhesive/ sealant compositions includes alkyl
ether cyanoacrylate. Alkyl ethyl cyanoacrylates have the gen-
eral formula:
NC R1 R2"
0/ \O/
wherein R1" is a straight, branched or cyclic alkyl, and R2" is
a straight, branched or cyclic alkyl group. Preferably, R1" is a
C1, C2 or C3 alkyl group, such as methyl or ethyl; and R2" is a
C1-C16 alkyl group, more preferably a C1-C10 alkyl group,
such as methyl, ethyl, propyl, isopropyl, butyl, isobutyl, pen-
tyl, hexyl, heptyl, octyl, nonylor decyl, and even more pref-
erably a C2, C3 or C4 alkyl group.
Examples of alkyl ether cyanoacrylates include, but are not
limited to, isopropyoxy ethyl cyanoacrylate (IPECA) and
methoxy butyl cyanoacrylate (MBCA) or combinations
thereof. IPECA may be represented by the formula above,
wherein R1" is ethylene and R2" is isopropyl. MBCA may be
represented by the formula above, wherein R1" is n-butylene
and R2" is methyl.
Alkyl ester cyanoacrylates and alkyl ether cyanoacrylates
are particularly useful for medical applications because of
their absorbability by living tissue and associated fluids. It is
desirable that 100% of the polymerized and applied
cyanoacrylate adhesive be absorbed in a period of less than 3
years, preferably approximately 1-24 months, more prefer-
ably 1-18 months, and most preferably 3-12 months after
application of the adhesive to living tissue. The absorption
time may vary depending on the particular uses and tissues
involved. It may be desirable for the absorption time to be
longer for some types of tissue and to be shorter for other
tissue types. For example, a longer absorption time may be
desired when the adhesive composition is applied to hard
tissues, such as bone, but a shorter absorption time may be
desired when the adhesive composition is applied to softer
tissues.
The selection of monomer will affect the absorption rate of
the resultant polymer, as well as the polymerization rate of the
monomer. Thus, two or more different monomers having
varied absorption and/or polymerization rates may be used in
combination to give a greater degree of control over the
US 7,932,305 B2
7
absorption rate of the resultant polymer, as well as the poly-
merization rate of the monomer. The adhesive composition
may comprise a mixture of monomer species with varying
absorption rates. Where two monomer species having differ-
ent absorption rates are used, it is preferred that the absorption
rates be sufficiently different that a mixture of the two mono-
mers can yield a third absorption rate that is effectively dif-
ferent from the absorption rates of the two monomers indi-
vidually. Compositions according to these embodiments are
described, for example, in U.S. Patent Publication No. 2002/
0037310 and U.S. Pat. No. 6,620,846, both incorporated
herein by reference in their entireties.
Suitable monomer compositions may be prepared by mix-
ing suitable quantities of an alkyl ot-cyanoacrylate such as
2-octyl ot-cyanoacrylate with one of butyl lactoyl cyanoacry-
late (BLCA), butyl glycoloyl cyanoacrylate (BGCA), isopro-
pyl glycoloyl cyanoacrylate (IPGCA), ethyl lactoyl
cyanoacrylate (ELCA), and ethyl glycoloyl cyanoacrylate
(EGCA). Such mixtures may range from ratios of about 90: 10
to about 10:90 by weight, preferably about 75:25 to about
25:75 by weight.
The stability of ot-cyanoacrylate monomer compositions
may be adversely affected by the use of certain amounts of
BLMA polymer as an absorbable viscosity modifying agent.
More particularly, in general, the higher the concentration of
BLMA polymer in an ot-cyanoacrylate monomer composi-
tion, the less stable the monomer composition may become.
To overcome the stability issue, a stabilizer or stabilizing
agent may be added to the composition to prevent premature
polymerization or to increase the shelf life of the 0t-cy-
anoacrylate monomeric composition. For example, boron tri-
fluoride may be used as a stabilizing agent. Other suitable free
radical stabilizing agents for use in monomeric 0t-cyanoacry-
late adhesive compositions include, but are not limited to,
hydroquinone, hydroquinone monomethyl ether, catechol,
pyrogallol, benzoquinone, 2-hydroxybenzoquinone, p-meth-
oxy phenol, t-butyl catechol, butylated hydroxy anisole, buty-
lated hydroxy toluene, and t-butyl hydroquinone and mix-
tures or combinations thereof. The free radical stabilizing
agents may be used in amounts from about 5 to about 10,000
ppm. In exemplary embodiments, if hydroquinone is used,
the amount may be from about 5 to about 70 ppm and may be
used in conjunction with butylated hydroxy amsole in an
amount of about 500 to about 10,000 ppm.
The ot-cyanoacrylate adhesive compositions may also
optionally include at least one anionic vapor phase stabilizer
and at least one anionic liquid phase stabilizer. Examples of
such anionic agents are described for example, in U.S. Pat.
No. 6,620,846, incorporated herein by reference in its
entirety.
The anionic vapor phase stabilizers may be selected from
among known stabilizers, including, but not limited to, sulfur
dioxide or hydrogen fluoride. Typically, each anionic vapor
phase stabilizer is added in such an amount to give a concen-
tration of less than about 200 parts per million (ppm). In
exemplary embodiments, each anionic vapor phase stabilizer
is present in an amount from about 1 to about 200 ppm,
preferably from about 10 to about 75 ppm, even more pref-
erably from about 10 to about 50 ppm, and most preferably
from about 10 to about 20 ppm.
The liquid phase anionic stabilizer is a very strong acid that
has an aqueous pKa of less than 1.0. Examples of such very
strong acids include, but are not limited to, sulfuric acid
(pKa-3.0), perchloric acid (pKa-5), hydrochloric acid (pKa-
7.0), hydrobromic acid (pKa-9), fluorosulfonic acid (pKa
Coments go here:
- Log in to post comments