Cyanoacrylate compositions with vinyl terminated ester groups
Cyanoacrylate compositions with vinyl terminated ester groups
US6174919
Company:
Year:
Type of document:
Language:
US006174919B1
(12) United States Patent (10) Patent No.: US 6,174,919 B1
Hickey (45) Date of Patent: Jan. 16, 2001
(54) CYANOACRYLATE COMPOSITIONS WITH 5,259,835 11/1993 Clark et al. .
VINYL TERMINATED ESTER GROUPS 5,328,687 7/ 1994 Leung Ct a1~ ~
5,480,935 * 1/1996 Greff et al. ........................ .. 524/776
(75) Inventor: Timothy P. Hickey, Raleigh, NC (US) 3 ~
(73) Assignee: Closure Medical Corporation, 53? E: 3‘ ‘
Raleigh’ NC (US) 5,624,669 4/1997 Leun: et al. .
( * ) Notice: Under 35 U.S.C. 154(b), the term of this FOREIGN PATENT DOCUMENTS
patent shall be extended for 0 days. 1527561 10/1978 (GB) .
wo 97/31598 9/1997 (wo) .
(21) Appl. No.: 09/025,473 * Cited by examiner
(22) Filed: Feb’ 18’ 1998 Primary Exami/1er—Thurman K. Page
(51) Int. Cl.7 ................................................... .. A01N 37/34 AssisramExaminer—P. Kulkosky . .
(52) U.s. Cl. ..................... .. 514/519, 156/326; 156/330.9; (74) Attorney Agenh or Flrm—0hff & Berrldge, PLC
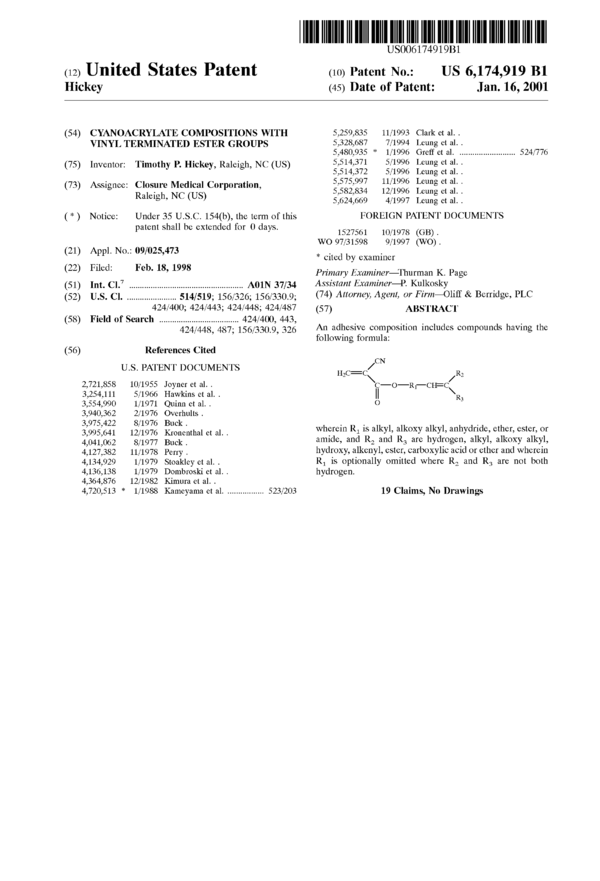
424/400; 424/443; 424/448; 424/487 (57) A]3,s'1‘RAC'1‘
(58) Field of Search ................................... .. 424/400, 443, . . . . .
424/448, 487; 156/3309, 326 An adhesive composition includes compounds having the
following formula:
(56) References Cited
U.S. PATENT DOCUMENTS H C_C CN R
2 j 2
2,721,858 10/1955 Joyner et al. . (:—o—R1—(:H=(:
3,254,111 5/1966 Hawkins et al. . || \R3
3,554,990 1/1971 Quinn et al. . 0
3,940,362 2/1976 Overhults.
3,975,422 8/1976 Buck. . . .
3,995,641 12/1976 Kmnenthal et al. . wherein R1 1S alkyl, alkoxy alkyl, anhydride, ether, ester, or
4,041,062 8/1977 Buck. amide, and R2 and R3 are hydrogen, alkyl, alkoxy alkyl,
4,127,332 11/1973 perry . hydroxy, alkenyl, ester, carboxylic acid or ether and wherein
4,134,929 1/1979 stoakley et a1. . R1 is optionally omitted where R2 and R3 are not both
4,136,138 1/1979 Dombroski et al. . hydrogen.
4,364,876 12/1982 Kimura et al. .
4,720,513 * 1/1988 Kameyama et al. ............... .. 523/203 19 Claims, No Drawings
US 6,174,919 B1
1
CYANOACRYLATE COMPOSITIONS WITH
VINYL TERMINATED ESTER GROUPS
BACKGROUND OF THE INVENTION
1. Field of Invention
The present invention is directed to monomer composi-
tions useful to form industrial, consumer or medical adhe-
sives and sealants, and methods of applying such composi-
tions. More particularly, this invention relates to monomeric
cyanoacrylate compositions having vinyl terminated ester
groups that allow a biologically acceptable method of cross-
linking through the vinyl group.
2. Description of Related Art
U.S. Pat. No. 5,624,669 to Leung et al., discloses hemo-
static procedures for sealing punctures and incisions in
blood vessels and internal organs by applying a cyanoacry-
late monomer. Although the cyanoacrylate may polymerize
and/or cross-link in vivo, it preferably does so without the
need for external sources of physical initiation such as
irradiation.
U.S. Pat. No. 4,134,929 to Stoakley et al. discloses a
polymerizable monomeric allyl 2-cyanoacrylate containing
portion comprising an amount of an organic peroxide free
radical providing compound sufficient to cause crosslinking
of a difunctional monomer diester with the allyl
2-cyanoacrylate. Stoakley discloses that crosslinking may
occur by way of the allyl group.
U.S. Pat. No. 4,136,138 to Dombroski et al. discloses a
polymerizable monomeric 2-cyanoacrylate containing por-
tion comprising an amount of an organic peroxide free
radical providing compound sufficient to cause crosslinking
of a difunctional monomer diester with the 2-cyanoacrylate.
Dombroski discloses that the allyl 2-cyanoacrylate-based
adhesive compositions are especially useful as dental adhe-
s1ves.
U.S. Pat. No. 3,975,422 to Buck discloses difunctional
monomers where R is an organic linking group derived from
a diol or a dihalide of the formula X—R—X, where X is
either Cl, Br, I, or hydroxy. The difunctional monomers are
employed as crosslinking agents for monofunctional esters
of 2-cyanoacrylates. The monofunctional cyanoacrylate
monomers may be cyanoacrylates that are terminated by an
alkyl, cyclohexyl or phenyl group. Copolymerized compo-
sitions of the monomer blends (difunctional and
monofunctional) are useful as adhesives in dental applica-
tions. The polymerization of these compositions is initiated
by an anionic catalyst or by thermal or other means.
SUMMARY OF THE INVENTION
The present invention is directed to monomeric
cyanoacrylate compositions having vinyl terminated ester
groups that cross-link through the vinyl group, and biomedi-
cal uses of such compositions. Cross-linking occurs by way
of the vinyl terminated ester groups. In embodiments,
chemical durability, flexibility and elasticity of the resulting
polymers or copolymers may be increased and degradability
can be reduced. In addition, in embodiments high tempera-
tures or ultraviolet initiators may not be needed for cross-
linking.
DETAILED DESCRIPTION OF PREFERRED
EMBODIMENTS
Cyanoacrylate adhesive compositions of the invention
contain compounds represented by the following formula
(1)1
10
15
20
25
30
35
40
45
50
55
60
65
2
(1)
CN
I‘I2C:C R2
fi?O*R1:CH:C\
R3
0
wherein R1 is alkyl, alkoxy, anhydride, ether, ester, or amide,
and R2 and R3 are independently alkyl, alkoxy, hydrogen,
hydroxy, alkenyl, ester, carboxylic acid, ether, or electron
withdrawing groups such as halogens, amides, cyanos,
esters, acids and ethers. Preferably, R1 is an alkyl having
from about 1 to 8 carbon atoms. Preferably, R2 and R3 are
hydrogen atoms. More preferably, R2 and R3 are alkyl
groups having from about 1 to 3 carbon atoms. The R1 group
extends the distance of the R2 and R3 groups away from the
carbonyl group, thereby making them more chemically
accessible and improving chemical durability, flexibility and
elasticity of a polymer comprising the monomer. In
embodiments, R1 may be omitted if R2 and R3 are not both
hydrogen.
In embodiments, the adhesive compositions may addi-
tionally contain heat and/or light (e.g., visible or ultraviolet
light) activated initiators and accelerators that initiate cross-
linking of the cyanoacrylate compounds.
Particular initiators for particular systems may be readily
selected by one of ordinary skill in the art without undue
experimentation. Suitable polymerization initiators for the
cyanoacrylate compositions include, but are not limited to,
detergent compositions; surfactants: e.g., nonionic surfac-
tants such as polysorbate 20 (e.g., Tween 20”‘), polysorbate
80 (e.g., Tween SOTM) and poloxamers, cationic surfactants
such as tetrabutylammonium bromide, anionic surfactants
such as benzalkonium chloride or its pure components,
stannous octoate (tin (II) 2-ethylheaxanoate), and sodium
tetradecyl sulfate, and amphoteric or zwitterionic surfactants
such as dodecyldimethyl(3-sulfopropyl)ammonium
hydroxide, inner salt; amines, imines and amides, such as
imidazole, tryptamine, urea, arginine and povidine;
phosphines, phosphites and phosphonium salts, such as
triphenylphosphine and triethyl phosphite; alcohols such as
ethylene glycol, methyl gallate, ascorbic acid, tannins and
tannic acid; inorganic bases and salts, such as sodium
bisulfite, magnesium hydroxide, calcium sulfate and sodium
silicate; sulfur compounds such as thiourea and polysulfides;
polymeric cyclic ethers such as monensin, nonactin, crown
ethers, calixarenes and polymeric epoxides; cyclic and acy-
clic carbonates, such as diethyl carbonate; phase transfer
catalysts such as Aliquat 336; and organometallics such as
cobalt naphthenate and manganese acetylacetonate and radi-
cal initiators.
Suitable initiators for both of the polymerization of the
cyanoacrylate and cross-linking of the vinyl group of the
composition include, but are not limited to, radicals, such as
di-t-butyl peroxide, azobisisobutyronitrile and benzoylper-
oxide and sodium bisulfite. The polymerizable and/or cross-
linkable material may also contain an initiator which is
inactive until activated by a catalyst or accelerator (included
within the scope of the term “initiator” as used herein).
Accelerators for radical initiators such as dimethylaminopy-
ridine and other aminopyridine type molecules may act as an
initiator for the cyanoacrylate as well as for the radical
polymerization of the vinyl moiety.
In embodiments, when R1 is omitted and R2 and/or R3 are
a moiety other than hydrogen, and the composition is to be
cationically polymerizable, materials such as strong acids,
US 6,174,919 B1
3
alkyl iodides (iodomethane), iodine, acetyl perchlorate, and
Lewis acids (boron trifluoride, tin tetrachloride, aluminum
trichloride, and organometallic derivatives, e.g., RAlCl2,
R2AlCl, wherein R is an alkyl group and R2 is two R groups)
may be used.
The monomer compositions of the present invention and
polymers formed therefrom are useful as tissue adhesives,
sealants for preventing bleeding or for covering open
wounds, and in other biomedical applications. They find
uses in, for example, apposing surgically incised or trau-
matically lacerated internal and/or external tissues; setting
fractured bone structures; retarding blood flow from
wounds; drug delivery; dressing burns; and aiding repair and
regrowth of living tissue.
Conventional surgical adhesive compositions have
included plasticizers with the adverse effect of reducing the
film strength. It has been discovered that, contrary to prior
belief, the film strength (e.g., toughness) under certain
conditions is not adversely reduced upon the addition of
greater amounts of plasticizing agent. Depending on the
particular acidic stabilizing agent and the purity of the
monomer utilized in the adhesive composition, the addition
of greater amounts of plasticizing agent may increase the
toughness of the resulting bond formed on the wound.
Acidic stabilizing agents do not significantly affect the
polymerization of the monomer in the present composition
and provide increased film strength with increasing amounts
of plasticizing agents.
Monomers that may be used in this invention are
polymerizable, e. g. anionically polymerizable or free radical
polymerizable, to form polymers. In embodiments, the
cyanoacrylate composition may comprise a homopolymer of
the monomer of formula (I) or a copolymer or terpolymer
with other monomers. Such other monomers include, but are
not limited to, acrylate monomers, methacrylate monomers,
and 1,1-disubstituted ethylene monomers of the formula:
(11)
wherein X and Y are each strong electron withdrawing
groups, and R is H, —CH=CH2, or an alkyl such as methyl,
ethyl and other lower alkyls such as butyl and the like,
provided that X and Y are both cyano groups, a C1-C4 alkyl
group.
Examples of monomers within the scope of formula (II)
include alpha-cyanoacrylates, vinylidene cyanides, C1-C4
alkyl homologues of vinylidene cyanides, dialkyl methylene
malonates, acylacrylonitriles, vinyl sulfinates and vinyl sul-
fonates of the formula H2C=CX‘Y‘ wherein X‘ is —SO2R‘
or —SO3R‘ and Y‘ is —CN, —COOR‘, —COCH3, —SO2R‘
or —SO3R‘, and R‘ is H or hydrocarbyl.
Preferred monomers of formula (II) for use in this inven-
tion are alpha-cyanoacrylates. These monomers are known
in the art and have the formula
HRC:CXY
(111)
CN
HR2C=C
COOR3,
wherein R2 is hydrogen or lower alkyl and R3 is a hydro-
carbyl or substituted hydrocarbyl group including polymeric
groups; a group having the formula —R4—O—R3—O—R6,
wherein R4 is a 1,2-alkylene group having 2-4 carbon
atoms, R3 is an alkylene group having 2-4 carbon atoms,
and R6 is an alkyl group having 1-6 carbon atoms; or a
group
10
15
20
25
30
35
40
45
50
55
60
65
having the formula R7ji|Z:O:R8, wherein R7 is
O
we
:CH2?, :CHZ, or C(CH3)2
wherein n is 1-10, preferably 1-5 carbon atoms and R8 is an
organic moiety.
Examples of suitable hydrocarbyl and substituted hydro-
carbyl groups include straight chain or branched chain alkyl
groups having 1-16 carbon atoms; straight chain or
branched chain C1-C16 alkyl groups substituted with an
acyloxy group, a haloalkyl group, an alkoxy group, a halo-
gen atom, a cyano group, or a haloalkyl group; straight chain
or branched chain alkenyl groups having 2 to 16 carbon
atoms; straight chain or branched chain alkynyl groups
having 2 to 12 carbon atoms; cycloalkyl groups; aralkyl
groups; alkylaryl groups; and aryl groups.
The organic moiety R8 may be substituted or unsubsti-
tuted and may be straight chain, branched or cyclic,
saturated, unsaturated or aromatic. Examples of such
organic moieties include C1-C8 alkyl moieties, C2-C8 alk-
enyl moieties, C2-C8 alkynyl moieties, C3-C12
cycloaliphatic moieties, aryl moieties such as phenyl and
substituted phenyl and aralkyl moieties such as benzyl,
methylbenzyl and phenylethyl. Other organic moieties
include substituted hydrocarbon moieties, such as halo (e.g.,
chloro-, fluoro- and bromo-substituted hydrocarbons) and
oxy- (e.g., alkoxy substituted hydrocarbons) substituted
hydrocarbon moieties. Preferred organic moieties are alkyl,
alkenyl and alkynyl moieties having from 1 to about 8
carbon atoms, and halo-substituted derivatives thereof. Par-
ticularly preferred are alkyl moieties of 4 to 6 carbon atoms.
In the cyanoacrylate monomer of formula (III), R3 is
preferably an alkyl group having 1-10 carbon atoms or a
group having the formula —AOR9, wherein A is a divalent
straight or branched chain alkylene or oxyalkylene moiety
having 2-8 carbon atoms, and R9 is a straight or branched
alkyl moiety having 1-8 carbon atoms.
Examples of groups represented by the formula —AOR9
include 1-methoxy-2-propyl, 2-butoxy ethyl, isopropoxy
ethyl, 2-methoxy ethyl, and 2-ethoxy ethyl.
The preferred alpha-cyanoacrylate monomers used in this
invention are 2-octyl cyanoacrylate, dodecyl cyanoacrylate,
2-ethylhexyl cyanoacrylate, butyl cyanoacrylate, methyl
cyanoacrylate, 3-methoxybutyl cyanoacrylate,
2-butoxyethyl cyanoacrylate, 2-isopropoxyethyl
cyanoacrylate, or 1-methoxy-2-propyl cyanoacrylate.
The alpha-cyanoacrylates of formula (III) can be prepared
according to methods known in the art. Reference is made,
for example, to U.S. Pat. Nos. 2,721,858 and 3,254,111, each
of which is hereby incorporated by reference herein. For
example, the alpha cyanoacrylates can be prepared by react-
ing an alkyl cyanoacetate with formaldehyde in a non-
aqueous organic solvent and in the presence of a basic
catalyst, followed by pyrolysis of the anhydrous intermedi-
ate polymer in the presence of a polymerization inhibitor.
The alpha-cyanoacrylate monomers prepared with low
moisture content and essentially free of impurities are pre-
ferred for biomedical use.
The alpha-cyanoacrylates of formula (III) wherein R3 is a
group having the formula —R4—O—R3—O—R6 can be
prepared according to the method disclosed in U.S. Pat. No.
4,364,876 to Kimura et al., which is hereby incorporated by
reference herein. In the Kimura et al. method, the alpha-
US 6,174,919 B1
5
cyanoacrylates are prepared by producing a cyanoacetate by
esterifying cyanoacetic acid with an alcohol or by transes-
terifying an alkyl cyanoacetate and an alcohol; condensing
the cyanoacetate and formaldehyde or para-formaldehyde in
the presence of a catalyst at a molar ratio of 0.5-1.521,
preferably 0.8-1.221, to obtain a condensate; depolymeriz-
ing the condensation reaction mixture either directly or after
removal of the condensation catalyst to yield crude
cyanoacrylate; and distilling the crude cyanoacrylate to form
a high purity cyanoacrylate.
The alpha-cyanoacrylates of formula (III) wherein R3 is a
group having the formula
—R7—C—o—R8
O
can be prepared according to the procedure described in U.S.
Pat. No. 3,995,641 to Kronenthal et al., which is hereby
incorporated by reference herein. In the Kronenthal et al.
method, such alpha-cyanoacrylate monomers are prepared
by reacting an alkyl ester of an alpha-cyanoacrylic acid with
a cyclic 1,3-diene to form a Diels-Alder adduct which is then
subjected to alkaline hydrolysis followed by acidification to
form the corresponding alpha-cyanoacrylic acid adduct. The
alpha-cyanoacrylic acid adduct is preferably esterified by an
alkyl bromoacetate to yield the corresponding carbalkoxym-
ethyl alpha-cyanoacrylate adduct. Alternatively, the alpha-
cyanoacrylic acid adduct may be converted to the alpha-
cyanoacrylyl halide adduct by reaction with thionyl
chloride. The alpha-cyanoacrylyl halide adduct is then
reacted with an alkyl hydroxyacetate or a methyl substituted
alkyl hydroxyacetate to yield the corresponding car-
balkoxymethyl alpha-cyanoacrylate adduct or carbalkoxy
alkyl alpha-cyanoacrylate adduct, respectively. The cyclic
1,3-diene blocking group is finally removed and the car-
balkoxy methyl alpha-cyanoacrylate adduct or the car-
balkoxy alkyl alpha-cyanoacrylate adduct is converted into
the corresponding carbalkoxy alkyl alpha-cyanoacrylate by
heating the adduct in the presence of a slight deficit of
maleic anhydride.
Examples of monomers of formula (III) include cyano-
pentadienoates and alpha-cyanoacrylates of the formula:
(IV)
CN
Hzc=c
COOR3
wherein Z is —CH=CH2 and R3 is as defined above. The
monomers of formula (IV) wherein R3 is an alkyl group of
1-10 carbon atoms, i.e., the 2-cyanopenta-2,4-dienoic acid
esters, can be prepared by reacting an appropriate
2-cyanoacetate with acrolein in the presence of a catalyst
such as zinc chloride. This method of preparing
2-cyanopenta-2,4-dienoic acid esters is disclosed, for
example, in U.S. Pat. No. 3,554,990, which is hereby
incorporated by reference herein.
Preferred monomers are alkyl alpha-cyanoacrylates and
more preferably octyl alpha-cyanoacrylates, especially
2-octyl alpha-cyanoacrylate. Monomers utilized in the
present application should be very pure and contain few
impurities (e.g., surgical grade).
Compositions of the present invention may include at
least one plasticizing agent that imparts flexibility to the
polymerized monomer formed on the wound or incision.
10
15
20
25
30
35
40
45
50
55
60
65
6
The plasticizing agent preferably contains little or no mois-
ture and should not significantly affect the polymerization of
the monomer.
Other compositions are exemplified by U.S. Pat. Nos.
5,259,835 and 5,328,687 and U.S. patent applications Ser.
Nos. 08/609,921, 08/714,288, 08/909,845, 08/755,007,
08/920,876, and 08/488,411, all incorporated by reference
herein in their entirety.
Examples of suitable plasticizers include acetyl tributyl
citrate, dimethyl sebacate, triethyl phosphate, tri(2-
ethylhexyl)phosphate, tri(p-cresyl)phosphate, glyceryl
triacetate, glyceryl tributyrate, diethyl sebacate, dioctyl
adipate, isopropyl myristate, butyl stearate, lauric acid,
trioctyl trimellitate, dioctyl glutarate and mixtures thereof.
Preferred plasticizers are tributyl citrate and acetyl tributyl
citrate. In embodiments, suitable plasticizers include poly-
meric plasticizers, such as polyethylene glycol (PEG) esters
and capped PEG esters or ethers, polyester glutarates and
polyester adipates.
Compositions of the present invention may also include at
least one acidic stabilizing agent that inhibits polymeriza-
tion. Such stabilizing agents may also include mixtures of
anionic stabilizing agents and radical stabilizing agents.
Examples of suitable anionic stabilizing agents include,
but are not limited to, sultones (e.g., ot-chloro-ot-hydroxy-
o-toluenesulfonic acid-y-sultone), sulfur dioxide, sulfuric
acid, sulfonic acid, sulfurous acid, lactone, boron trifluoride,
organic acids, alkyl sulfate, alkyl sulfite, 3-sulfolene,
alkylsulfone, alkyl sulfoxide, mercaptan, and alkyl sulfide
and mixtures thereof. Preferable anionic stabilizing agents
are acidic stabilizing agents of organic acids such as acetic
acid or phosphoric acid. In embodiments, the amount of
sulfur dioxide stabilizer is less than 100 ppm, preferably
5-75 ppm, and more preferably from about 20-50 ppm. The
amount of sultone and/or trifluoracetic acid is about
500-3000 ppm.
Examples of suitable radical stabilizing agents include
hydroquinone, hydroquinone monomethyl ether, catechol,
pyrogallol, benzoquinone, 2-hydroxybenzoquinone,
p-methoxy phenol, t-butyl catechol, butylated hydroxy
anisole, butylated hydroxy toluene, and t-butyl hydro-
quinonc.
Suitable acidic stabilizing agents include those having
aqueous pKa ionization constants ranging from -12 to 7,
preferably from about -3.5 to about 6, and more preferably
from about 2 to about 5.5. For example, suitable acidic
stabilizing agents include: hydrogen sulfide (pKa 7.0), car-
bonic acid (pKa 6.4), triacetylmethane (pKa 5.9), acetic acid
(pKa 4.8), benzoic acid (pKa 4.2), 2,4-dinitrophenol (pKa
4.0), formic acid (pKa 3.7), nitrous acid (pKa 3.3), hydrof-
luoric acid (pKa 3.2), chloroacetic acid (pKa 2.9), phospho-
ric acid (pKa 2.2), dichloroacetic acid (pKa 1.3), trichloro-
acetic acid (pKa 0.7), 2,4,6-trinitrophenol (picric acid) (pKa
0.3), trifluoroacetic acid (pKa 0.2), sulfuric acid (pKa _3.0),
and mixtures thereof.
When adding the above-mentioned acidic stabilizing
agents to the adhesive composition, the addition of plasti-
cizing agents in amounts ranging from about 0.5 wt. % to
about 16 wt. %, preferably from about 3 wt. % to about 9 wt.
%, and more preferably from about 5 wt. % to about 7 wt.
% provides increased film strength (e.g., toughness) of the
polymerized monomer over polymerized monomers having
amounts of plasticizing agents and acidic stabilizing agents
outside of the above ranges.
The concentration of the acidic stabilizing agents utilized
may vary depending on the strength of the acid. For
example, when using acetic acid, a concentration of 80-200
US 6,174,919 B1
7
ppm (wt/wt), preferably 90-180 ppm (wt/wt), and more
preferably 100-150 ppm (wt/wt) may be utilized. When
using a stronger acid such as phosphoric acid, a concentra-
tion range of 20-80 ppm (wt/wt), preferably, 30-70 ppm
(wt/wt) and more preferably 40-60 ppm (wt/wt) may be
utilized. In embodiments, the amount of trifluoroacetic acid
is about 100 to 3000 ppm, preferably 500-1500 ppm. In
other embodiments, the amount of phosphoric acid is about
10-200 ppm, preferably about 50-150 ppm, and more
preferably about 75-125 ppm.
Other compositions are exemplified by U.S. Pat. Nos.
5,624,669, 5,582,834, 5,575,997, 5,514,371, 5,514,372,
5,259,835 and 5,328,687, incorporated by reference herein
in their entirety. The compositions of the present invention
may also include at least one biocompatible agent effective
to reduce active formaldehyde concentration levels pro-
duced during in vivo biodegradation of the polymer (also
referred to herein as “formaldehyde concentration reducing
agents”). Preferably, this component is a formaldehyde
scavenger compound. Examples of formaldehyde scavenger
compounds useful in this invention include sulfites;
bisulfites; mixtures of sulfites and bisulfites; ammonium
sulfite salts; amines; amides; imides; nitriles; carbamates;
alcohols; mercaptans; proteins; mixtures of amines, amides,
and proteins; active methylene compounds such as cyclic
ketones and compounds having a b-dicarbonyl group; and
heterocyclic ring compounds free of a carbonyl group and
containing an NH group, with the ring made up of nitrogen
or carbon atoms, the ring being unsaturated or, when fused
to a phenyl group, being unsaturated or saturated, and the
NH group being bonded to a carbon or a nitrogen atom,
which atom is directly bonded by a double bond to another
carbon or nitrogen atom.
Bisulfites and sulfites useful as the formaldehyde scav-
enger compound in this invention include alkali metal salts
such as lithium, sodium and potassium salts, and ammonium
salts, for example, sodium bisulfite, potassium bisulfite,
lithium bisulfite, ammonium bisulfite, sodium sulfite, potas-
sium sulfite, lithium sulfite, ammonium sulfite, and the like.
Examples of amines useful in this invention include the
aliphatic and aromatic amines such as, for example, aniline,
bcnzidinc, aminopyrimidinc, tolucnc-diaminc,
triethylenediamine, diphenylamine, diaminodiphenylamine,
hydrazines and hydrazide.
Suitable proteins include collagen, gelatin, casein, soy-
bean protein, vegetable protein, keratin and glue. The pre-
ferred protein for use in this invention is casein.
Suitable amides for use in this invention include urea,
cyanamide, acrylamide, benzamide, and acetamide. Urea is
a preferred amide.
Suitable alcohols include phenols, 1,4-butanediol,
d-sorbitol, and polyvinyl alcohol.
Examples of suitable compounds having a b-dicarbonyl
group include malonic acid, acetylacetone, ethylacetone,
acetate, malonamide, diethylmalonate or another malonic
ester.
Preferred cyclic ketones for use in this invention include
cyclohexanone or cyclopentanone.
Examples of suitable heterocyclic compounds for use as
the formaldehyde scavenger in this invention are disclosed,
for example, in U.S. Pat. No. 4,127,382 (Perry) which is
hereby incorporated by reference herein. Such heterocyclic
compounds include, for example, benzimidazole, 5-methyl
benzimidazole, 2-methylbenzimidazole, indole, pyrrole, 1,2,
4-triazole, indoline, benzotriazole, indoline, and the like.
Apreferred formaldehyde scavenger for use in this inven-
tion is sodium bisulfite.
10
15
20
25
30
35
40
45
50
55
60
65
8
In practicing the present invention, the formaldehyde
concentration reducing agent, e.g., formaldehyde scavenger
compound, is added in an effective amount to the cyanoacry-
late. The “effective amount” is that amount sufficient to
reduce the amount of formaldehyde generated during sub-
sequent in vivo biodegradation of the polymerized
cyanoacrylate. This amount will depend on the type of active
formaldehyde concentration reducing agent, and can be
readily determined without undue experimentation by those
skilled in the art.
The formaldehyde concentration reducing agent may be
used in this invention in either free form or in microencap-
sulated form. Other compositions are exemplified by U.S.
patent application Ser. No. 08/714,288, incorporated by
reference herein in their entirety.
When microencapsulated, the formaldehyde concentra-
tion reducing agent is released from the microcapsule con-
tinuously over a period of time during the in vivo biodeg-
radation of the cyanoacrylate polymer.
For purposes of this invention, the microencapsulated
form of the formaldehyde concentration reducing agent is
preferred because this embodiment prevents or substantially
reduces polymerization of the cyanoacrylate monomer by
the formaldehyde concentration reducing agent, which
increases shelf-life and facilitates handling of the monomer
composition during use.
Microencapsulation of the formaldehyde scavenger can
be achieved by many known microencapsulation techniques.
For example, microencapsulation can be carried out by
dissolving a coating polymer in a volatile solvent, e.g.,
methylene chloride, to a polymer concentration of about 6%
by weight; adding a formaldehyde scavenger compound in
particulate form to the coating polymer/solvent solution
under agitation to yield a scavenger concentration of 18% by
weight; slowly adding a surfactant-containing mineral oil
solution to the polymer solution under rapid agitation;
allowing the volatile solvent to evaporate under agitation;
removing the agitator; separating the solids from the mineral
oil; and washing and drying the microparticles. The size of
the microparticles will range from about 0.001 to about 1000
microns.
The coating polymer for microcncapsulating the formal-
dehyde concentration reducing agent should be polymers
which undergo in vivo bioerosion, preferably at rates similar
to or greater than the cyanoacrylate polymer formed by the
monomer, and should have low inherent moisture content.
Such “bioerosion” can occur as a result of the physical or
chemical breakdown of the encapsulating material, for
example, by the encapsulating material passing from solid to
solute in the presence of body fluids, or by biodegradation
of the encapsulating material by agents present in the body.
Examples of coating materials which can be used to
microencapsulate the formaldehyde concentration reducing
agent include polyesters, such as polyglycolic acid, poly-
lactic acid, poly-1,4-dioxa-2-one, polyoxaltes,
polycarbonates, copolymers of polyglycolic acid and poly-
lactic acid, polycaprolactone, poly-b-hydroxybutyrate,
copolymers of epsilon-caprolactone and delta-valerolactone,
copolymers of epsilon-caprolactone and DL-dilactide, and
polyester hydrogels; polyvinylpyrrolidone; polyamides;
gelatin; albumin; proteins; collagen; poly(orthoesters); poly
(anhydrides); poly(alkyl-2-cyanoacrylates); poly
(dihydropyrans); poly(acetals); poly(phosphazenes); poly
(urethanes); poly(dioxinones); cellulose; and starches.
Examples of the surfactant which can be added to the
mineral oil include those commercially available under the
designations Triton x-100, Tween 20 and Tween 80.
US 6,174,919 B1
9
The composition of this invention may further contain one
or more adjuvant substances, such as thickening agents,
medicaments, or the like, to improve the medical utility of
the monomer for particular medical applications.
Suitable thickeners include, for example,
polycyanoacrylates, polylactic acid, polyglycolic acid,
lactic-glycolic acid copolymers, polycaprolactone, lactic
acid-caprolactone copolymers, poly-3-hydroxybutyric acid,
polyorthoesters, polyalkyl acrylates, copolymers of alky-
lacrylate and vinyl acetate, polyalkyl methacrylates, and
copolymers of alkyl methacrylates and butadiene. Examples
of alkyl methylacrylates and acrylates are poly(2-ethylhexyl
methacrylate) and poly(2-ethylhexyl acrylate), also poly
(butylmethacrylate) and poly(butylacrylate), also copoly-
mers of various acrylate and methacrylate monomers, such
as poly(butyl methacrylate-co-methylacrylate).
To improve the cohesive strength of adhesives formed
from the compositions of this invention, difunctional mono-
meric cross-linking agents may be added to the monomer
compositions of this invention. Such crosslinking agents are
known. Reference is made, for example, to U.S. Pat. No.
3,940,362 to Overhults, which is hereby incorporated by
reference herein. Examples of suitable crosslinking agents
include alkyl bis(2-cyanoacrylates), triallyl isocyanurates,
alkylene diacrylates, alkylene dimethacrylates, trimethylol
propane triacrylate, and alkyl bis(2-cyanoacrylates). A cata-
lytic amount of an amine activated free radical initiator may
be added to initiate polymerization of the cyanoacrylate
monomer/crosslinking agent blend.
The compositions of this invention may further contain
fibrous reinforcement and colorants, i.e., dyes and pigments.
Examples of suitable fibrous reinforcement include PGA
microfibrils, collagen microfibrils, cellulosic microfibrils,
and olefinic microfibrils. Examples of suitable colorants
include 1-hydroxy-4-[4-methylphenyl-amino]-9,10
anthracenedione (D+C violet No. 2); disodium salt of
6-hydroxy-5-[(4-sulfophenyl)axo]-2-naphthalene-sulfonic
acid (FD+C Yellow No. 6); 9 -(o-carboxyphenyl)-6-
hydroxy-2,4,5,7-tetraiodo-3H-xanthen-3-one, disodium salt,
monohydrate (FD+C Red No. 3); 2-(1,3-dihydro-3-oxo-5-
sulfo-2H-indol-2-ylidene)-2,3-dihydro-3-oxo-1H-indole-5-
sulfonic acid disodium salt (FD+C Blue No. 2); and
[phthalocyaninato (2-)] copper.
Depending on the particular requirements of the user, the
adhesive compositions of this invention can be applied by
known means such as with a swab, glass stirring rod, sterile
brush or medicine dropper. However, in many situations a
spray dispensing package is preferred in which the adhesive
composition is in solution with a compatible anhydrous
propellant. Other modes of application are exemplified in
U.S. patent application Ser. No. 08/488,411, incorporated by
reference herein in its entirety.
What is claimed is:
1. An adhesive composition with improved properties of
chemical durability, flexibility and elasticity of resulting
polymers and copolymers, comprising a compound of the
following formula (I):
(1)
CN
H2C:C R2
C*O*R ‘ CH1 C
II 1 \
R3
wherein R1 is selected from the group consisting of alkyl
having at least 2 carbon atoms, alkoxy, anhydride,
10
15
20
25
30
35
40
45
50
55
60
65
10
ether, ester, and amide, wherein R2 and R3 are inde-
pendently selected from the group consisting of
hydrogen, alkyl, alkoxy, hydroxy, alkenyl, ester, car-
boxylic acid, ether and electron withdrawing groups,
and
wherein R1 may also optionally be omitted or be an alkyl
having 1 carbon atom when R2 and R3 are not both
hydrogen.
2. The adhesive composition according to claim 1,
wherein said electron withdrawing groups are selected from
the group consisting of halogens, amides, cyanos, esters,
acids and ethers.
3. The adhesive composition according to claim 1,
wherein R1 is an alkyl having from about 2 to 8 carbon
atoms.
4. The adhesive composition according to claim 1,
wherein R2 and R3 are hydrogen.
5. The adhesive composition according to claim 1,
wherein R2 and R3 are alkyls having from 1 to 3 carbon
atoms.
6. The adhesive composition according to claim 1, further
comprising an initiator.
7. The adhesive composition according to claim 6,
wherein said initiator is selected from the group consisting
of benzalkonium chloride, stannous octoate and sodium
tetradecyl sulfate.
8. The adhesive composition according to claim 1, further
comprising a radical initiator.
9. The adhesive composition according to claim 8,
wherein said radical initiator is selected from the group
consisting of di-t-butyl peroxide, azobisisobutyronitrile and
benzoylperoxide.
10. A method of joining together surfaces, comprising:
(a) holding together at least two surfaces to form abutted
surfaces, and
(b) applying across said abutted surfaces an adhesive
composition according to claim 1.
11. An adhesive composition comprising a homopolymer
of the compound of claim 1.
12. An adhcsivc composition comprising a copolymcr of
the compound of claim 1 and a 1,1-disubstituted ethylene
monomer.
13. The adhesive composition according to claim 12,
wherein said ethylene monomer is n-butyl cyanoacrylate or
2-octyl cyanoacrylate.
14. The adhesive composition according to claim 1,
wherein crosslinking occurs through the vinyl terminated
ester group.
15. The adhesive composition according to claim 1,
further comprising an ultraviolet initiator.
16. A method of treatment comprising using the adhesive
composition of claim 1 in a biomedical application selected
from the group consisting of drug delivery, burn treatment,
setting fractured bone structures, retarding blood flow from
wounds, aiding repair and regrowth of living tissue and
apposing surgically incised or traumatically lacerated inter-
nal or external tissues.
17. The adhesive composition according to claim 1,
further comprising at least one acidic stabilizing agent.
18. The adhesive composition according to claim 17,
further comprising at least one radical stabilizing agent.
19. The adhesive composition according to claim 18,
further comprising at least one plasticizing agent.
* * * * *
Coments go here:
- Log in to post comments