Combined Use of an Alkaline Earth Metal Compound and a Sterilizing Agent to Maintain Osteoinduction Properties of a Demineralized Bone Matrix
Combined Use of an Alkaline Earth Metal Compound and a Sterilizing Agent to Maintain Osteoinduction Properties of a Demineralized Bone Matrix
US7771652
Company:
Year:
Abstract:
A method is disclosed that produces allografts from matrices typically containing demineralized bone matrix (DBM) powder, demineralized bone matrix gel, demineralized bone matrix paste, bone cement, cancellous bone, or cortical bone and mixtures thereof. The matrices are sterilized utilizing supercritical CO2 in the presence of a sterilizing additive and an entrainer such as an alkaline earth metal compound, preferably CaCO3. The resultant allograft materials have a reduced rate of rejection when used in allograft procedures including, bone, cartilage, tendon, and ligament grafting procedures.
Type of document:
Language:
(12) United States Patent
Christopher et al.
IJS007771652B2
US 7,771,652 B2
Aug. 10, 2010
(10) Patent No.:
(45) Date of Patent:
(54) COMBINED USE OF AN ALKALINE EARTH
METAL COMPOUND AND A STERILIZING
AGENT TO MAINTAIN OSTEOINDUCTION
PROPERTIES OF A DEMINERALIZED BONE
MATRIX
(75) Inventors: Renee A. Christopher, Dryden, NY
(US); J. Anastasia Nichols, Ithaca, NY
(US)
(73) Assignee: Novasterilis, Inc., Lansing, NY (US)
( * ) Notice: Subject to any disclaimer, the term of this
patent is extended or adjusted under 35
U.S.C. 154(b) by 214 days.
(21) Appl.No.: 12/101,489
(22) Filed: Apr. 11, 2008
(65) Prior Publication Data
US 2009/0257914 A1 Oct. 15, 2009
(51) Int. Cl.
A61L 2/20 (2006.01)
(52) U.s. Cl. ...................................................... .. 422/33
C02
Cylinder
Valve
C02
Booster
Compressor
(58) Field of Classification Search ................. .. 422/28,
422/31, 33
See application file for complete search history.
(56) References Cited
U.S. PATENT DOCUMENTS
7,108,832 B2 *
* cited by examiner
Primary Examiner—Walter D Griffin
Assistant Examiner—Timothy Cleveland
(74) Attorney, Agent, or Firm—Welsh & Flaxman LLC
9/2006 Christensen et al. ........ .. 422/33
(57) ABSTRACT
A method is disclosed that produces allografts from matrices
typically containing demineralized bone matrix (DBM) pow-
der, demineralized bone matrix gel, demineralized bone
matrix paste, bone cement, cancellous bone, or cortical bone
and mixtures thereof. The matrices are sterilized utilizing
supercritical CO2 in the presence of a sterilizing additive and
an entrainer such as an alkaline earth metal compound, pref-
erably CaCO3. The resultant allograft materials have a
reduced rate of rejection when used in allograft procedures
including, bone, cartilage, tendon, and ligament grafting pro-
cedures.
10 Claims, 2 Drawing Sheets
U.S. Patent Aug. 10, 2010 Sheet 1 012 US 7,771,652 B2
N mm H
E
8
8
S we
ow 8
av
_
4
>
> 1%,“.
- 8 mm
mm
26>
Eam
mm
E
H mm
\/o_
smmuaeou
._<
Emocm 3 3
mm Nag
_%____»o
%> N8
I w
R mm
U.S. Patent Aug. 10, 2010 Sheet 2 of2 US 7,771,652 B2
Percentsunnval
U1 0'!
o o
c o
C: c:
30.00 1 - —
20.00 —— -—*~—~—~— - ~—-~ —~»-
1000 -—-~h~——
0.00 ~ f~——w 0 —-——.
0 50 100 150 200 250 300
Time (mins)
Fig. 3
US 7,771,652 B2
1
COMBINED USE OF AN ALKALINE EARTH
METAL COMPOUND AND A STERILIZING
AGENT TO MAINTAIN OSTEOINDUCTION
PROPERTIES OF A DEMINERALIZED BONE
MATRIX
FIELD OF THE INVENTION
The present invention relates to methods of reducing rejec-
tion in allograft procedures including, bone, cartilage, ten-
don, and ligament grafting procedures.
BACKGROUND OF THE INVENTION
Allograft bone is a graft substitute readily available from
cadavers and avoids the surgical complications and subject
morbidity associated with harvesting autologous bone.
Allograft bone is essentially a load-bearing matrix comprised
of cross-linked collagen, hydroxyapatite, and osteoinductive
Bone Morphogenetic Proteins (BMP). Human allograft tis-
sue is widely used in orthopedic surgery. Allograft tissue is
strong, integrates with the recipient host bone, and can be
shaped either by the surgeon to fit the specific defect or
shaped commercially by a manufacturing process. Allograft
bone is available in two basic forms: cancellous and cortical.
Cortical bone is a highly dense structure comprised of triple
helix strands of collagen fiber reinforced with hydroxyapa-
tite. The hydroxyapatite component is responsible for the
high compressive strength and stiffness of bone while the
collagen fiber component contributes to its elastic nature, as
well as torsional, shear, and tensile strength. Cortical bone is
the main load-bearing component of long bones in the human
body.
Use of allograft material is often a preferred treatment
option for musculoskeletal related injuries. Significant prob-
lems associated with the use of allografts are recipient rejec-
tion of the tissue due to the presence of donor related antigens
still present in the allograft, allograft inability to incorporate
into the host, and contaminated tissue used in transplant.
Numerous methodologies have been employed to reduce the
level of antigenic compounds present in allografts and per-
form low-level disinfection and sterilization. These methods
generally include numerous washes with a variety of chemi-
cals. These methods have extreme drawbacks to the extent
that they utilize reactive chemicals that alter the structural
components of the allograft or denature and/ or remove com-
pounds that facilitate integration of the allograft by the recipi-
ent.
It therefore would be highly desirable if a sterilization
method could be provided leading to improved clincal out-
comes of bone grafting.
Recently, in U.S. Pat. No. 6,149,864 to Dillow et al and
U.S. Pat. No. 7,108,832 to Christensen et al. (the entire con-
tent of both are expressly incorporated herein by reference),
the use of supercritical CO2 was disclosed as an altemative to
existing technologies for sterilizing a wide range of products
for the healthcare industry with little or no adverse effects on
the material treated.
SUMMARY OF THE INVENTION
The present invention fills the foregoing need by providing
devices, systems and methods for repairing bone and soft
tissue defects in a subject, wherein the bone and soft tissue
defects are repairable by a bone or soft tissue graft procedure.
The present invention contemplates the use of allografts that
have been produced from matrices typically containing dem-
10
15
20
25
30
35
40
45
50
55
60
65
2
ineralized bone matrix (DBM) powder, demineralized bone
matrix gel, demineralized bone matrix paste, bone cement,
cancellous bone or cortical bone sterilized utilizing super-
critical CO2 in the presence of a sterilizing additive and an
alkaline earth metal compound such as CaCO3.
These and other aspects and advantages will become more
apparent after careful consideration is given to the following
detailed description of the preferred exemplary embodiments
thereof.
BRIEF DESCRIPTION OF THE
ACCOMPANYING DRAWINGS
Reference will hereinafter be made to the accompanying
drawings, wherein like reference numerals throughout the
various FIGURES denote like structural elements, and
wherein;
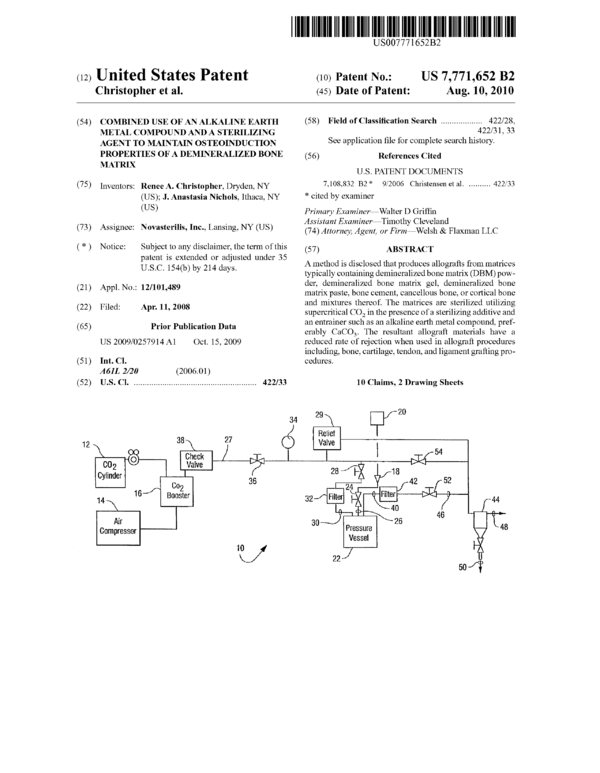
FIG. 1 is a schematic view of a presently preferred steril-
ization apparatus in accordance with the present invention;
FIG. 2 is a detailed schematic view of the pressure vessel
employed in the apparatus of FIG. 1; and
FIG. 3 is a graph that relates the survival percentage of
endospores to processing time in the apparatus of FIG. 1.
DETAILED DESCRIPTION OF THE INVENTION
The present invention contemplates the production of
allografts from matrices typically containing one or more
material of: demineralized bone matrix (DBM) powder, dem-
ineralized bone matrix gel, demineralized bone matrix paste,
bone cement, cancerous bone, cortical bone, or mixtures
thereof and the like; that are sterilized utilizing supercritical
CO2 in the presence of a sterilizing additive and an entrainer
such as an alkaline earth metal compound, preferably CaCO3.
The resultant allograft materials display a reduced rate of
rejection when used in bone allograft. The sterilization appa-
ratus and methods of the present invention are usefully
employed to sterilize a variety of materials to produce
allografts including but not limited to demineralized bone
matrix powder, demineralized bone matrix gel, demineral-
ized bone matrix paste, bone cement, cancellous bone and
cortical bone and the like. The instant invention can be prac-
ticed with different bone structures. For example, suitable
bone graft structures may include cartilage, cortical bone,
cancellous bone, subchondral bone, and any combination of
the various bone tissue types. In addition, bone-tendon-bone
allografts used for ACL reconstruction and structures
employed for long bone allograft tumor reconstruction can
also be used. The graft structure may comprise a composite
bone that includes a polymer and a demineralized bone, and,
optionally, a bone powder. These compounds may be used in
different ratios that can be determined by a person of ordinary
skill in the art. A non-limiting example of the suitable com-
posite bone includes 50% polylactide (PLA), 30% deminer-
alized bone (
Coments go here:
- Log in to post comments