����������������(19) United States
US 20030164222A1
(12) Patent Application Publication (10) Pub. No.: US 2003/0164222 A1
Kneafsey et al.
(43) Pub. Date: Sep. 4, 2003
(54) SEMI-SOLID PRIMER COMPOSITIONS
(76) Inventors: Brendan J. Kneafsey, Dublin (IE);
John Guthrie, Kildare (IE)
Correspondence Address:
Henkel Loctite Corporation
1001 Trout Brook Crossing
Rocky Hill, CT 06067 (US)
(21) Appl. No.: 10/276,421
(22) PCT Filed: May 30, 2001
(86) PCT No.: PCT/IE01/00070
(30) Foreign Application Priority Data
May 31, 2000 (IE) ......................................... .. 2000/0439
Publication Classification
(51) Int. Cl.7 ...................................................... .. C09J 5/04
(52) U.S. Cl. ........................... .. 156/314; 524/387; 401/55
(57) ABSTRACT
A composition comprising at least one carrier solvent, at
least one primer component and at least one gelling or
solidifying agent is provided. The composition according to
the invention may he provided in the form of a soft solid, for
example, in the form of a stick. A two-part adhesive system
is also provided, comprising at least one anaerobic product
and a composition comprising at least one carrier solvent, at
least one primer component Where the primer component is
a cure promoting agent and at least one gelling or solidifying
agent. A two-part adhesive system comprising a cyanoacry-
late adhesive and a composition having at least one carrier
solvent, at least one primer component and at least one
gelling or solidifying agent is also provided. A pack is
provided comprising a shaped mass of a composition which
includes at least one carrier solvent, at least one primer
component and at least one gelling or solidifying agent, and
a container (1) for the composition which has a mechanism
for expelling the shaped mass.
Patent Application Publication Sep. 4, 2003 US 2003/0164222 A1
US 2003/0164222 A1
SEMI-SOLID PRIMER COMPOSITIONS
FIELD OF THE INVENTION
[0001] The present invention relates to semi-solid primer
compositions, particularly well suited to promoting the
bonding of substrates, such as those constructed of non-
polar or highly crystalline materials or for promoting the
cure of otherwise slow curing products.
BRIEF DESCRIPTION OF RELATED
TECHNOLOGY
[0002] Primers have long been used to promote the bond-
ing of substrates which otherwise are not readily bonded
together to a satisfactory extent by adhesive alone. The
primer promotes adhesion to the surface. Materials which do
not bond satisfactorily with adhesive alone are often referred
to as “difficult-to-bond”. Where both surfaces to be bonded
are difficult-to-bond, both may be primed prior to attempted
adhesion. Primer is applied to the surfaces to be bonded, to
“activate” or “prime” the surface for reception of later
applied adhesive. Certain primer components are thus often
referred to as adhesion-promoting agents” or “adhesion-
promoting primers” as they can promote subsequent adhe-
sion of substrates with a conventional adhesive.
[0003] The types of surface (substrate) which are difficult-
to-bond with adhesives, especially with cyanoacrylate adhe-
sives, are widely recognised and include non-polar or highly
crystalline substrates.
[0004] Non-polar substrates typically have low surface
energy which is due to an absence of polar groups on the
surface to be bonded. Polar groups are generally thought to
raise the surface energy to the level needed to give satis-
factory adhesion with conventional adhesives.
[0005] Non-polar substrates are often constructed of poly-
olefins in particular linear polymers of simple olefins, such
as polyethylene, polypropylene, polybutene, polytetrafluo-
roethylene and the like, including their copolymers; poly-
acetals, plasticised PVC (polyvinyl chloride), polyurethane,
EPDM (ethylene-propylene diamine monomer) rubber, ther-
moplastic rubbers based on vulcanised polyolefins and the
like.
[0006] Difficult-to-bond materials which are widely rec-
ognised include those with a waxy or paraffin-like surface
character; a low critical surface tension of wetting; and
which may have at their surface a weak boundary layer.
[0007] It will be appreciated therefore, that the main
function of an adhesion-promoting primer is to promote
adhesion in cases where curing (polymerization) occurs
readily but where adhesion may not take place at all or to a
desired extent. As stated above this a scenario typical of
cyanoacrylate adhesives.
[0008] A second function of a primer composition may be
to promote cure of the curable composition. Primer compo-
nents which have this function are referred to herein as
“cure-promoting”. In some instances curable compositions
may provide a desired degree of adhesion but achieve
adhesion at an undesired rate. For example the curable
composition may cure to give sufficient adhesion but may do
so only very slowly so that [text missing or illegible
when filed] bonded must be kept in contact over a period
of hours or even longer. While relatively long cure times are
Sep. 4, 2003
desirable in some instances, a shorter cure time without
deleteriously affecting bond strength is desirable for many
other purposes. Slow cure is a property associated with
many products including those termed “anaerobic” products.
These are polymerizable products which are stable and
remain unpolymerized in the presence of air (oxygen in
particular) and which tend to cure rapidly in the absence of
air.
[0009] It is normal for an initiator component of a cure
system to be excluded from the primer composition so that
the primer composition is absent an initiator component.
[0010] It is known to provide cure-promoting primer com-
positions for the purpose of decreasing cure times. In such
cases the cure-promoting primer is applied (usually by
application in solution form as described below) to the
desired substrate and the curable composition is then applied
to the surface treated with primer. The cure-promoting
primer acts to decrease cure times by accelerating the cure
of the composition but without for example diminishing
bond strength of adhesives, or sealing capability of sealants.
[0011] It is possible that any given primer may have dual
functionality i.e. promoting adhesion and promoting cure
though it is usual for primers to be classified as having one
of these main functions.
[0012] The present invention is concerned with primers in
general including both adhesion-promoting, and cure-pro-
moting primers.
[0013] Application of a primer composition which com-
prises a primer component in a solvent to a substrate to be
primed is typically by way of a brush or some such other
applicator, to form a layer or coating on the surface. The
coating is then typically dried, or allowed to dry for a period,
before bonding with adhesive is attempted, and to ensure
that the surface is properly primed. In this respect at least for
adhesion-promotion primers the solvent is normally a vola-
tile one i.e. evaporates readily at room temperature leaving
behind the primer component. The solvent does not there-
fore interfere with the [text missing or illegible when
filed] adhesion process—acting only as an inert carrier
solvent for placing the adhesion-promoting primer compo-
nent on the substrate. For cure-promoting primers the sol-
vent may be a polymerizable monomer or oligomer which
may take part in the polymerization process during bonding.
Accordingly these solvents are not usually considered
“inert” in the sense that the participate in the subsequent
bonding process. They do however act as carrier solvents for
the primer component. Monomeric or oligomeric solvents
do not tend to evaporate to any appreciable extent after
application so that they are not normally considered volatile.
These compositions will most usually be liquid also.
[0014] The nature of such primer compositions can result
in difficult handling for example unwanted spillage, run-on
of the coating applied, and the cumbersome task of having
to use an applicator or brush. It is desirable to provide a more
easily handled and potentially safer form of primer compo-
sition.
[0015] Primer compositions typically include at least one
priming compound or agent which is the primer component
of the composition. Various primer components and primer
compositions are described below.
[0016] It is desirable therefore to provide a primer com-
position in a consumer-friendly manner to allow for ease of
US 2003/0164222 A1
handling etc. Presenting primer compositions in consumer-
friendly forms and/or packages is a difficult task as the
primer component must not have its adhesion- or cure-
promoting capabilities deleteriously affected by the form, or
package, in which it is presented for use.
[0017] Various types of adhesive compositions are avail-
able commercially ranging from low viscosity (liquid) com-
positions to gels and other medium viscosity compositions
to higher viscosity products such as pastes, and soft-solid
products.
[0018] It is known to thicken or gel adhesive such as
cyanoacrylate adhesives with polymethylmethacrylate or
thixotropic agents such as those described in U.S. Pat. Nos.
4,447,607 and 4,533,422 (Litke).
[0019] It is also known for instance to formulate adhesives
as “stick” compositions. The patent literature on stick adhe-
sives is extensive and covers a broad range of adhesive
types, from emulsion adhesives through solvent based adhe-
sives, to contact adhesives, as well as gelling and solidifying
additives for the preparation of the sticks ranging from
thermosetting through natural polymers to inert fillers. A
well known example of one such adhesive stick sold under
the trade name PrittStickTM by Henkel KGaA which is an
emulsion-based adhesive.
[0020] In the patent literature the following patents typify
the type of composition in which adhesive components have
been formulated as soft-solids, and more particularly sticks.
U.S. Pat. No. 5,433,775 discloses an adhesive stick consist-
ing of a water based preparation of starch derivatives and a
soap gel as the shaping gel-forming component. See also
U.S. Pat. No. 5,371,131.
[0021] U.S. Pat. No. 3,846,363 relates to an adhesive
crayon composition containing a sorbitol-benzaldehyde
reaction product as an additive. U.S. Pat. No. 4,639,475
discloses an adhesive stick composed of the reaction product
of sorbitol and/or xylitol and benzaldehyde as the gel-
forming composition together with an adhesive resin which
is the reaction product of methyl vinyl ether/maleic acid
anhydride copolymers with partially neutralised acid phos-
phate esters of non-ionic wetting agents of the lower alky-
lene oxide adduct type.
[0022] Japanese unexamined patent application laid open
(Kokai) 51-103939 describes a stick-like epoxy adhesive
and a stick-like epoxy hardening agent which is used there-
with. The sticks are obtained by suitably compounding
gelling agent or/and water is and/or organic solvent with
liquid or solution type epoxy adhesive and epoxy hardening
agent.
[0023] DE 199 57 677 A1, published after the priority date
of the present application, of Henkel KGaA describes a
cyanoacrylate adhesive, coating or sealing material which
also contains at least one condensation product of an alde-
hyde or ketone with a polyol. [text missing or illegible
when filed] may be in stick form.
[0024] While a wide variety of sticks have been described,
no prior publication has suggested that a primer composition
could be made in stick form at all, let alone while main-
taining its primer functionality. It would therefore be advan-
tageous to provide a primer composition in stick form.
SUMMARY OF INVENTION
[0025] The present invention provides a primer composi-
tion suitable for formulation/presentation in stick form. The
Sep. 4, 2003
primer composition may promote subsequent bonding of
non-polar or highly crystalline substrates such as polyole-
fins, or similar plastics substrates with low surface energy,
with adhesive such as cyanoacrylate adhesives. Alterna-
tively or additionally to being adhesion-promoting, the
composition may act as a cure-promoting agent to promote
cure of the curable product depending on the primer com-
ponent chosen. The compositions allow for ease of applica-
tion and/or handling, and allow for accuracy in application
without running on of the primer composition.
[0026]
[0027]
[0028] (a) at least one carrier solvent;
In particular the present invention provides:
a composition including:
[0029] (b) at least one primer component; and
[0030] (c) at least one gelling or solidifying agent.
[0031] The composition is easily cast in stick form and is
very useful in his respect. The present invention thus pro-
vides primer compositions typically for use with an adhe-
sive. In particular the adhesion-promoting primer composi-
tions of the present invention have been demonstrated as
being effective adhesion-promoting agents for example as a
cyanoacrylate primer composition which is particularly use-
ful for promoting adhesion of polyolefins. The cure-promot-
ing compositions within the present invention have been
shown to be effective as cure-promoting agents particularly
with [text missing or illegible when filed] adhesives
and sealants.
[0032] More particularly, the present invention relates to
the preparation and use of primer compositions in stick
form, to promote the bonding of non-polar or highly crys-
talline substrates with adhesives, especially with cyanoacry-
late adhesives or alternatively to promote cure of curable
products in particular to promote the cure of anaerobic
products.
[0033] The curing or polymerization of a film of adhesive
such as cyanoacrylate, between two materials, does not
necessarily result in a strong bond, particularly if the mate-
rials are non-polar, and if the polymerization of the adhesive
is excessively rapid, a very weak bond or no bond will result.
The adhesion-promoting compositions of the present inven-
tion help to alleviate this non-bonding problem. The cure-
promoting compositions of the present invention have been
found to promote (accelerate) curing of otherwise slow
curing products.
[0034] Suitably the composition is the form of a soft-solid.
The composition may be formulated in stick form, for
example by casting.
[0035] Suitably component (a) the carrier solvent com-
prises an organic solvent in which the primer component is
stable. Component (a) may be an organic solvent such as
ethanol, methanol, acetone, methyl ethyl ketone, 1,1,1-
trichloroethane, 1,1,2-trichloro-1,2,2,-trifuoroethane or mix-
tures thereof with each other or with other solvents such as
an azeotropic mixture of 1,1,2-trichloro-1,2,2,-trifluoroet-
hane with acetone. Desirably the solvent is ethanol. The
solvent is desirably an inert carrier solvent where the primer
composition is an adhesion-promoting one.
[0036] The function of the solvent is to provide a suitable
concentration of the primer component so that a coating of
US 2003/0164222 A1
selected thickness can be applied to a surface. Therefore, any
common solvent in which the primer component is stable
will suffice. The solvent will usually dissolve the primer
component though dissolving is not essential provided the
solvent acts to provide a dilute (and thus disperse) form of
the primer component for subsequent application. Certain
active primer components used in this invention [text
missing or illegible when filed] adhesion on non-polar
and highly crystalline substrates While certain others pro-
mote curing of curable products such as anaerobic adhe-
s1ves.
[0037] Component (c) is suitably the condensation prod-
uct of the reaction of at least one aldehyde and/or ketone
with a polyol.
[0038] Suitable polyols include those with at least one of
a 1,2-diol, 1,3 diol or 1,4 diol structure. The diols may
additionally have other groups such as ether, acid, amido,
cyano, hemiacetal or halogen. Examples of suitable polyols
include 1,2-ethandiol, 1,3-propandiol, 1,2-propandiol, 2,3-
butandiol, 1,4-butandiol, 2,2-dimethyl-1,3-propandiol, 2,2-
bis(hydroxymethyl)-1,3-propandiol, 2-(bromomethyl)-2-
(hydroxymethyl)-1,3-propandiol, 1,3,4-butantriol,
1-phenyl-1,2,3-propantriol, 1,2-hexandiol, neopentylglycol,
1,4-bishydroxymethylcyclohexane, 2-methyl-1,3-propan-
diol, hexantriol-(1,2,6), 2-(2-hydroxyethoxy)butan-1,3,4-
triol, glycerine, di and polyglycerine, diglycerindiacetate,
trimethylolpropane, di-(trimethylolpropane), trimethylole-
thane, pentaerythritol, bicyclo [2.2.1] heptane-2,3,5,6-tetrol,
2,2,3,3-tetrahydroxybutandiacid, dipentaerythritol, sorbitol,
formitol, xylitol, inositol, glucitol, glucose, saccharose/su-
crose, starch, cellulose, ascorbic acid, partially or fully
hydrolysed polyvinylacetate, 9,10-dihydroxy stearic acid
methyl ester, diacetylsorbitol and methylglyceride.
[0039] Most suitable polyols are sorbitol, xylitol and man-
nitol especially sorbitol.
[0040] Suitable aldehyde or ketones include those which
have at least one substituted or unsubstituted aromatic,
heteroaromatic or alicylic ring. These polyols may have
additional groups such as ether ester, amide, cyano and
halogen.
[0041] Examples of ketones include cyclopentanone,
cyclohexanone, cycloheptanone, 1-(3,3-dimethylcyclo-
hexyl)-ethanone, 1-cyclopropylethanone, 3-methyl-5-pro-
pylcyclohex-2-en-1-one, dicyclopropylmethanone, 4-tert-
butylcyclohexanone, dicyclohexylmethanone,
4-methylcyclohexanone, 1-(1-methylcyclopropyl)-etha-
none, (4-chlorophenyl)-cyclopropyl-methanone, 1-(1H-pyr-
rol-2-yl)-ethanone, 1-(2,4,6-trimethylphenyl)-ethanone,
1-(2-furanyl)-2-propanone, 1-(2-naphthalenyl)-ethanone,
1-(2-thienyl)-1-propanone, 1-(4-bromophenyl)-ethanone,
1-(4-methoxyphenyl)-ethanone, [text missing or illeg-
ible when filed] alenyl)-ethanone, 1,1-diphenyl-2-pro-
panone, 1,2-diphenylethanone, 1,3-diphenyl-2-propanone,
1-phenyl-1-butanone, 1-phenyl-1-decanone, 1-phenyl-1-
dodecanone, 1-phenyl-1-hexanone, 1-phenyl-1-octanone,
1-phenyl-1-pentanone, 1-phenyl-1-penten-3-one, 1-phenyl-
1-tetradecanone, 1-phenyl-2-butanone, 1-phenyl-2-pro-
panone, 1-pyrazinyl-ethanone, 2,2,2-trifluoro-1-phenyl-
ethanone, 1-(2-furanyl)-ethanone, 1-(2-pyridinyl)-ethanone,
1-(2-thienyl)-ethanone, 4-chloro-1-(4-fluorophenyl)-1-bu-
tanone, 4-phenyl-2-butanone, 1-phenyl-ethanone, bis-(2-hy-
droxyphenyl)-methanone, bis-(4-chlorophenyl)-methanone,
cyclopentylphenylmethanone, cyclopropyl(4-methoxyphe-
Sep. 4, 2003
nyl)-methanone, cyclopropyl-(4-methylphenyl)-methanone,
cyclopropyl-2-thienyl-methanone, cyclopropylphenyl-
methanone, 1,5 -diphenyl-1,4-pentadien-3-one, phenyl-2-py-
ridinyl-methanone, 2-bromo-1-(4-nitrophenyl)-ethanone,
2-naphthalenylphenyl-methanone, 3-chloro-1-phenyl-1-pro-
panone, 4-(4-hydroxyphenyl)-2-butanone, 4-(4-methox-
yphenyl)-3-buten-2-one, 1-(4-pyridinyl)-ethanone, 1-(4-hy-
droxyphenyl)-ethanone, 1-phenyl-1-propanone, 4-phenyl-3-
buten-2-one, diphenylmethanone, 1-phenyl-2-butanone,
1-phenyl-2-buten-1-one, bis-(4-methylphenyl)-methanone,
2-methyl-1-phenyl-1-propanone, 2-chloro-1-phenyl-etha-
none, cyclopropyl-(4-fluorophenyl)-methanone, 1-(p-meth-
oxyphenyl)-2-propanone, cyclohexylphenylmethanone and
phenyl-(2-thienyl)-methanone.
[0042] The following aldehydes are exemplary for use in
the present invention: benzaldehyde, 3-chlorobenzaldehyde,
4-chlorobenzaldehyde, 2,6-dichlorobenzaldehyde, 2,4-dini-
trobenzaldehyde, 3,4-dichlorobenzaldehyde, 3-fluoroben-
zaldehyde, 4-bromobenzaldehyde, 2-methyltetrahydroben-
zaldehyde, tetrahydrobenzaldehyde, 2-methyl-5-
isopropylcyclopenten-1-aldehyde, 2,2,4-
trimethylcyclohexa-4,6-dien-1-aldehyde, 3(4)-methyl-1-
propylcyclohexen-3-aldehyde, 1,3(4)-dimethylcyclohexen-
3-aldehyde, 2-methyl-1-propylcyclohexen-3-aldehyde,
3-cyclohexen-1-aldehyde, 2,3,4,5,6-pentafluorobenzalde-
hyde, 2,4,6-trihydroxybenzaldehyde, 4-tolylacetaldehyde,
2-methylbenzaldehyde, 4-hydroxybenzaldehyde, 3-methyl-
benzaldehyde, 2-hydroxy-1-naphthalaldehyde, 4-methyl-
benzaldehyde, 3,5-dimethoxy-4-hydroxybenzaldehyde, cin-
nam-aldehyde, 3-nitrobenzaldehyde,
2-pentylcinnamaldehyde, 4-diethylaminobenzaldehyde,
4-methoxybenzaldehyde, 2-phenylpropionaldehyde,
2-methoxycinnamaldehyde, 4-methylbenzaldehyde, phe-
noxyacetaldehyde, methylpyrrol-2-aldehyde, 2,5-dimethox-
ytetrahydrofuran-3-aldehyde, 2,5-dipropyl-3,4-dihydropy-
ran-2- , [text missing or illegible when filed] 2,5-
diethyl-3,4-dihydropyran-2-aldehyde, 2,5-diisopropyl-3,4
dihydropyran-2-aldehyde, 2,5-dimethyl-3,4-dihydropyran-
2-aldehyde, 2,5-dibutyl-3,4-dihydropyran-2-aldehyde,
thiophen-3-aldehyde, indol-3-aldehyde, pyridine-3-alde-
hyde, pyridine-4-aldehyde and n-methylpyrrole-2-aldehyde.
[0043] Desirable aldehydes include benzaldehyde, 3-chlo-
robenzaldehyde and 3-fluorobenzaldehyde.
[0044] Particular acetals and ketals include: di-O-ben-
zylidenmannitol, di-O-(2-chlorobenzylidene)mannitol,
di-O-(4-nitrobenzylidene)mannitol, di-O-(3-fluoroben-
zylidene)mannitol, O-benzylidenesorbitol, di-O-benzylide-
nesorbitoldiacetate, di-O-(2-chlorobenzylidene)sorbitoldi-
acetate, tri-O-(4-chlorobenzylidene)sorbitol,
O-benzylidenethreitol, O-benzylidene tartaric acid methyl-
ester, O-cyclohexylidenglycerine, O-cyclohexylidene ascor-
bic acid and O-benzylidene-9, 10-dihydroxy stearic acid
methylester.
[0045] Suitably the aldehyde is benzaldehyde, 3-chlo-
robenzaldehyde or 3-fluorobenzaldehyde especially benzal-
dehyde. Suitably the polyol is sorbitol, xylitol or mannitol
especially sorbitol. The condensation product may be di-O-
benzylidene mannitol; di-O-(3-fluorobenzylidene) mannitol
or di-O-benzylidene sorbitol especially di-O-benzylidene
sorbitol.
[0046] Component (c) the gelling or solidifying agent is
useful for the preparation of a stick from the solutions
containing the adhesion-promoting agent.
[0047] Suitable gelling agents for inclusion as component
(c) include acetals of sugars, particularly acetals of sorbitol,
US 2003/0164222 A1
which are particularly effective as gelling agents. One such
gelling agent includes di-O-dibenzylidene sorbitol (also
referred to simply as dibenzylidene sorbitol) sold by
Roquette Freres, France under the trademark DisorbeneTM.
Other acetals such as those described above have also been
found to be useful.
[0048] Acetals of sugars, particularly natural sugars, for
example acetals of sorbitol, [text missing or illegible
when filed] previously used as gelling agents for the
preparation in stick form and these materials are useful in the
present invention.
[0049] Suitably component (c) the gelling or solidifying
agent has a concentration in the solvent from about 0.01%
to about 20%, such as about 0.01% to about 15%, typically
from about 0.05% to about 10% for example about 0.1% to
about 5% by weight by weight based on the total weight of
the composition.
[0050] It is desirable that a solution of the active primer
component in the solvent should have a concentration of
about 0.001 to about 30% by weight based on the total
weight of the composition for example from about 0.01 to
20% by weight based on the total weight of the composition.
Suitable concentrations are about 0.005 to about 15% such
as about 0.005 to about 10% by weight based on the total
weight of the composition for example about 0.001 to about
5% by weight based on the total weight of the composition
such as 0.01 to about 2% by weight based on the total weight
of the composition.
[0051] Desirably the primer component is an adhesion-
promoting agent.
[0052] Suitably (where adhesion-promotion is desired) the
primer component is selected from:
[0053] 1,5-diazabicyclo[4.3.0]non-5-ene having the
formula:
/
N
[0054] 1,8-diazabicyclo[5.4.0]undec-7-ene having the for-
mula:
N
/
[0055] or 1,5,7-triazabicyclo[4.4.0]dec-5-ene having the
formula:
NH N
U/J
Sep. 4, 2003
[0056] (ii) triphenyl phosphine, or
[0057] (iii) ethylenediamine or derivatives of ethylenedi-
amine according to the formula:
R R
\ /
N— CH2— CH2—N
/
R R
[0058] wherein each R, which may be the same or differ-
ent, represents hydrogen, an alkyl, alkenyl or alkoxy group
having 1 to 8 carbon atoms, an aryl group having 6 to 8
carbon atoms, a nitrogen-, sulphur-, or silicon-substituted
group having up to 8 carbon atoms or a heterocyclic group
having up to 8 carbon atoms which may be unsubstituted or
substituted with hydroxy, ether oxygen or sulphur; or
[0059] (iv) an imidazole having the formula:
R1
[0060] wherein R1 may be hydrogen; an alkyl group that
is unsubstituted or substituted with an OH group or with an
(alkyl-O)3Si group, wherein the alkyl radical has 1 to 4
carbon atoms; an aryl-alkyl group with 7 to 10 carbon atoms;
or an imidazole-CO group, and R2 may be hydrogen or an
alkyl, aryl, or aryl-alkyl group with up to 17 carbon atoms,
with the condition that one of the substituents R1 or R2 has
an aromatic character and X is one of the groups
CH—CH or CR3R4 CRSR5 wherein R3, R4, R5
and R6 independently of one another represent hydrogen, an
alkyl group with 1 to 4 carbon atoms, or an aryl-alkyl group
with up to 17 carbon atoms.
[0061] Suitably (again particularly where adhesion pro-
motion is sought) the primer component (component
contains at least two nitrogen atoms. Desirably the primer
component is a compound of the group above, in par-
ticular 1,8-diazabicyclo[5.4.0]undec-7-ene.
[0062] From among the ethylenediamines labelled as
primer component (iii) above, suitable ethylenediamines are
those according to the formula give above wherein each R,
which may be the same or different, represents hydrogen, an
alkyl, alkenyl or alkoxy group, having 1 to 8 carbon atoms,
an aryl group having 6 to 8 carbon atoms, a nitrogen- or
sulphur-substituted group having 1 to 8 carbon atoms or a
heterocyclic group having up to 8 carbon atoms which may
be unsubstituted or substituted with hydroxy, ether oxygen
or sulphur.
[0063] Desirably ethylenediamine primer components are
those wherein at least one R is aryl, or each R is other than
hydrogen and at least one R is other than methyl.
[0064] Desirably the composition comprises as primer
components one or more of: N,N,N‘,N'-tetraethylethylene-
diamine and at least one of N‘-benzyl-N,
US 2003/0164222 A1
N‘dimethylethylenediamine,
N‘phenylethylenediamine,
dimethylethylenediamine or
aminopropyl-bis(2-ethylhexoxy)-silane.
N,N-diethyl-
N,N'-dibenzyl-N,N‘-
N-2-aminoethyl-3-
[0065] Examples of the primer component labelled as (iv)
above include the imidazole compounds include: those
derivatives where X is —CR3R4—CR3R6- especially 4,5
dihydroimidazole; these compounds may also be referred to
as imidazolines.
[0066] Desirable primer components include those imida-
zole compounds referred to above where X is —CR3R4—
CR3R6—, R1) R2) R3), R4, R3 and R6 desirably have the
following significance: R1 is hydrogen or a hydroxyalkyl
group, R2 is an aryl or an aryl-alkyl group with to 17 carbon
atoms and R3, R4, R3, and R6 independently are each
hydrogen or an alkyl group with 1 to 4 carbon atoms. More
desirably R1 is hydrogen or a hydroxyethyl group, R2 is a
benzyl or phenyl group, and R3, R4, R3 and R6 are hydrogen.
A particularly advantageous imidazoline derivative for use
in the invention is 2-phenyl-[text missing or illegible
when filed]
[0067] Typical examples of aryl or aralkyl groups that can
be the substituent R2 are phenyl, naphthyl, tolyl, xylyl,
benzyl, and naphthylmethyl groups. Typical examples of
alkyl groups with 1 to 17 carbon atoms that can likewise
form the R2 group are methyl, ethyl, propyl, butyl, pentyl,
heptyl, nonyl, undecyl, tridecyl, pentadecyl, and heptadecyl
groups; straight chain alkyl groups are most useful. In
addition, mixtures of these 2-alkylimidazole derivatives can
also be used, containing alkyl groups of different chain
lengths from the above list. Typical examples of alkyl
groups with 1 to 4 carbon atoms and aryl groups that can
form the substituents R3, R4, R3 and R6 can be obtained from
the above list.
[0068] When X is —CH=CH—, R1 and R2 desirably
have the following significance; R1 is an aryl group with 7
to 10 carbon atoms or an imidazole-CO group and R2 is
hydrogen or an alkyl group with 1 to 4 carbon atoms. Most
desirably R1 is a methyl or benzyl group and R2 is hydrogen
or a methyl group.
[0069] Other imidazole primer compounds useful in the
present invention include those of the Formula:
[0070] wherein R1 is a C1-C4 alkyl group optionally sub-
stituted with a phenyl group, and R2 is hydrogen or a C1-C4
alkyl group.
[0071] Desirably the composition when solidified has the
consistency of a soft-solid or waxy mass. The Theological
properties of the mass of product should be such that the
mass has a stable geometric shape. It is desirable that the
shaped mass e.g. a stick, is applicable by manual rubbing
against the substrate to which it is to be applied. The soft or
semi-solid mass should be shearable under relatively modest
Sep. 4, 2003
manual pressure to [text missing or illegible when
filed]ease of application. Under shear forces the solid may
liquify to form a film (or smear) of the composition on the
surface. It is desirable that the shaped mass retains its shape,
for example when stored at 20° C. for a number of days for
example at least about 10 days, more desirably for a number
of weeks or months. The composition exemplified herein
have proven to be stable over a number of months in such
conditions. The cast composition has the ease of handling
advantages of a completely solid material yet remains easily
dispensable. PrittStickTM is one commercially available
semi-solid mass sold as an adhesive stick which is well
known as a dispensable adhesive.
[0072] The primer composition composed of one of the
above primer components as a solution in a (solidified)
solvent is normally of such concentration that the thickness
of the coating can be controlled to achieve the full effects of
this invention.
[0073] The present invention also provides a composition
described above wherein the primer component is a cure-
promoting agent. Suitably the cure-promoting primer com-
ponent is selected from substituted thioureas, a compound
having a sulphur-containing free radical source, a compound
containing an oxidisable transition metal or a compound
containing one of the groups
[0074] Most desirable are compositions wherein the
primer component is a compound containing an oxidisable
transition metal. The transition metal is desirably selected
from those elements in the groups (columns) of the Periodic
Tables containing Cr, Mn, Fe, Co or Cu. The transition metal
may be selected from Cu, Cr, Co, Fe, and Mn, more
particularly Cu(I), Cu(II), Co(II), Mn(II), Mn(III) or Cr(II).
Particularly useful are those where the transition metal is
selected from Cu(I) or Cu(II), especially those compounds
which are salts of these particular forms of Cu, for example
salts of carboxylic acids, beta diketones or beta keto esters.
Particular salts are Cu(II) 2-ethylhexanoate or Cu(II) acety-
lacetone.
[0075] Where the primer component is a cure-promoting
primer for anaerobic products [text missing or illegible
when filed] may be a polymerizable monomer or polymer-
izable oligomer for example alkyl methacrylate esters such
as methyl or ethyl methacrylate. In the embodiment the
polymerisable monomer or oligomer may interact with the
polymerization process and thus form part of the cured
product. The monomer or oligomer should provide the
desired concentration of primer component. The solvent
may thus for example be methyl or ethyl methacrylate.
[0076] The invention also provides a two-part adhesive
system comprising at least one cyanoacrylate adhesive
and (ii) an adhesion-promoting primer composition accord-
ing to the present invention as defined above. Suitably the
cyanoacrylate is an alpha cyanoacrylate.
[0077] Suitable cyanoacrylate adhesives for use with the
adhesion-promoting primer of this invention are represented
by the general formula
US 2003/0164222 A1
H2C= C— COOR1
CN
[0078] wherein R1 is alkyl, alkenyl, cycloalkyl, aryl,
alkoxyalkyl, aralkyl, haloalkyl or another suitable group.
The lower alkyl alpha-cyanoacrylates are preferred, and in
particular methyl, ethyl, n-propyl, n-butyl, isobutyl, isopro-
pyl, allyl, cyclohexyl, methoxyethyl or ethoxyethyl
cyanoacrylates are preferred. Many alpha cyanoacrylates
can be obtained commercially as one component instant
adhesives, in which form they may be used in a method of
assembly of this invention.
[0079] The present invention also provides a two-part
adhesive system comprising at least one anaerobic prod-
uct and (ii) a cure-promoting composition as described
above. Suitable anaerobic products are described below.
[0080] The invention also relates to the solidified product
of a composition as described above. Suitably the compo-
sition is solidified in a desired geometric form, for example
in a cylindrical shape. Any suitable shape which allows for
ease or handling is desired and such shapes are typically
referred to as sticks. One method of preparing a soft-solid or
semi solid mass of the composition includes the steps of:
[0081] heating a composition comprising (a) at least
one carrier solvent; (b) at least one primer component; and
(c) at least one gelling or solidifying agent, to a desired
temperature; and
[0082] (ii) allowing the composition to cool or cooling the
composition sufficiently to set the composition.
[0083] Typically the composition will set (solidify to a
soft-solid) at a temperature of below about 30° C. for
example at about 15-22° C.
[0084] The invention also relates to a shaped mass pre-
pared by the method just described and particularly a mass
shaped in a stick form.
[0085] While these commercially available cyanoacry-
lates are composed principally of alpha cyanoacrylate mono-
mer, the formulation may contain stabilisers, thickeners,
adhesion-promoters, plasticizers, dyes, heat resistant addi-
tives, impact resistance modifiers, perfumes, diluents and
such like. These adjustments may also be used with anaero-
bic products.
[0086] It will be appreciated that if the composition is cast
before cooling to its set temperature then it will take the
shape of the container or mould in which it is cast. It is
desirable that the composition be cast in a desired geometric
shape for example as a stick for example a stick of a
generally cylindrical shape. The person skilled in the art will
appreciate that the pre-and post-casting composition will
have essentially the same compositional make-up, with
mainly physical changes from liquid to solid occurring
during casting. Little or no solvent will be lost during the
casting process. The amounts of the various components
thus remains essentially unchanged as between the liquid
and solidified compositions. No appreciable volume change
occurs during casting.
Sep. 4, 2003
[0087] In a further aspect the invention provides a method
of bonding a first (non-polar) [text missing or illegible
when filed] to a second substrate (which may be polar or
non-polar), which comprises treating (by priming) the first
(non-polar) substrate(s) by applications of a composition
according to the present invention, applying a cyanoacrylate
(suitably an alpha cyanoacrylate) adhesive to the treated
surface(s) and contacting the substrates for sufficient time
(and with sufficient pressure) to allow them to bond to each
other. In this way a bonded assembly is created. In the case
where the substrates to be bonded are both non-polar or
otherwise difficult-to-bond materials of that type, both sub-
strates may be primed with a composition of the invention.
The composition may suitably be applied by (manually)
rubbing the primer stick on the substrate. If the non-polar
substrate is to be bonded to a polar or more active substrate,
only the non-polar substrate needs to have applied the
primer. Following application of the primer to the non-polar
substrate(s), the bond may be completed using cyanoacry-
late adhesive in the normal manner. This method is espe-
cially useful for priming polyolefin substrates. This method
may also be used with anaerobic products where the sub-
strate to which the product is applied is not necessarily a
non-polar one. Anaerobic products may seal between rather
than bond together two substrates.
[0088] The invention relates also to a bonded assembly
created by this method. The invention thus discloses the use
of a primer composition in the manufacture of a castable
primer stick composition for example a primer stick for
priming substrates (for subsequent bonding with adhesive).
The adhesion-promoting compositions of the invention may
be used to prime non-polar or highly crystalline substrates.
[0089] The invention also provides an easy to use and
consumer-friendly pack comprising:
[0090] a shaped mass of a composition according to the
invention (a cast composition); and
[0091] (ii) a container for the composition, the container
having a mechanism for expelling the shaped mass. Desir-
ably the shaped mass is moveable between a position where
the shaped mass projects from the container, and a position
where the shaped mass is substantially located (retracted)
within the container.
[0092] The composition may be cast directly in the con-
tainer. Normally the container [text missing or illegible
when filed]bular and most often of cylindrical shape. The
container may be of the type having a displaceable mecha-
nism for example a carrier for displacing the mass of the
composition relative to the container. The carrier may move
the mass so that it projects from the container, or retract the
mass so that it is housed within the container. In this way the
mass may be extended for application to a substrate or
retracted for storage. The container may comprise a cap for
protection of the mass when the composition is not in use.
Desirably the container has means for manual adjustment of
the position of the mass or carrier, for example where the
carrier is reciprocally threaded on a winding mechanism and
can thus be extended or retracted by rotation of the winding
mechanism in one of two directions.
[0093] It will be appreciated by those skilled in the art that
a multitude of suitable containers may be used with the
shapes masses or sticks of the present invention. Typically
used containers are those with propulsion mechanisms to
propel the stick out of the container. Many such containers
are known for example for deodorants/anti-perspirants, and
make-up such as lipstick etc.
US 2003/0164222 A1
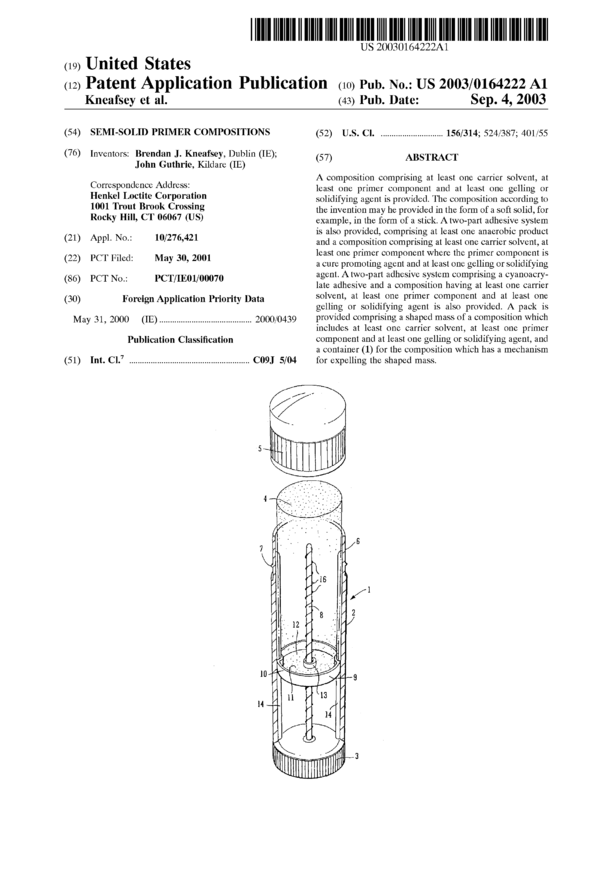
BRIEF DESCRIPTION OF THE FIGURES
[0094] FIG. 1 is a side (part-sectional) elevational view of
a container suitable for holding the composition of the
present invention; and
[0095] FIG. 2 is a top View of a carrier which forms part
of the container of FIG. 1.
DETAILED DESCRIPTION OF THE
INVENTION
[0096] Many primer components and primary composi-
tions have been developed for use in bonding of substrates,
in particular difficult-to-bond substrates as described above.
Some primers components and primer compositions are now
discussed. In accordance with the present invention the
primer components disclosed below in the documents dis-
cussed may be taken and formulated in a composition of the
present invention for casting in stick form.
[0097] For example, Japanese patent application Nos.
52-98062, 56-141328 and 57-119929 suggest the use of
primers prepared by dissolving a chlorinated polyethylene or
chlorinated polypropylene or a fatty acid modified acrylated
alkyd resin in an organic acid.
[0098] Primer based or organometallic compound are also
known. For instance, European patent application no. 0 129
069 A2 discloses the use of a primer comprising at least one
organometallic compound, for use with alpha cyanoacrylate
adhesives, which is useful in bonding non-polar substrates.
Japanese patent application No. 61023630 proposes orga-
nometallic primers for joining plastic models. U.S. Pat. Nos.
5,292,364, 5,110,392 and 4,818,325 also disclose the use of
primer compositions containing organometallic compounds
for use with cyanoacrylate adhesives.
[0099] Primers for polyolefins comprising modified or
grafted polyolefins such as chlorinated polyolefins, ethylene/
vinyl acetate copolymer or ethylene/propylene terpolymer,
(ii) a crosslinking binder and (iii) crosslinking agents, are
disclosed in European application No. 0 187 171A. German
application No. DE 3601518-A suggests the use of primers
for polyolefins comprising an organometallic compound and
an organic polymer; the organometallic compound being a
metal alcoholate and the term “metal” including metalloids,
phosphorous, boron or silicon atoms Japanese application
No. 61136567 describes a titanate primer for bonding
polypropylene.
[0100] The English language abstract [from JAPIO data-
base, accession no.1987-018486] for Japanese application
No. 60157940 discloses a primer composition based on a
solution of 4-vinyl pyridine as an essential ingredient, and
3,4-lutidine as an optional ingredient, for bonding polyole-
fins, polyacetal, polyamide, polyester, boron-polymer, sili-
cone or PVC.
[0101] The Derwent Abstract [Accession no.
19942559135] for Japanese patent application JP 610 0839
discloses dialkylamino diazabicycloundecene primer com-
positions for use with cyanoacrylate adhesives for plastics
bonding. For example polyolefins and EPDM. The com-
pounds, are those of formula I below
Sep. 4, 2003
[0102] (R, R1=C1_14 alkyl, benzyl).
[0103] U.S. Pat. No. 5,314,562 describes a method for
bonding substrates, one of which is a plastic, comprising (a)
applying to the plastic substrate a primer solution of an
adhesion-promoting ethylenediamine compound dissolved
in a solvent, (b) allowing the primer solution to dry, (c)
applying an alpha cyanoacrylate adhesive to one of the
substrates and (d) joining the substrates. The active adhe-
sion-promoting agent is dissolved in a solvent and applied as
a primer solution.
[0104] German patent publication no. DE 40 35 680
describes imidazole derivatives which are useful as adhe-
sion-promoting agents.
[0105] German patent publication DE 40 17 802 A1 also
relates to imidazole derivatives which are useful primers for
inclusion in the compositions of the present invention. In
particular the document discloses primer compounds of the
Formula:
N
E A
2
N R
R1
[0106] wherein R1 is a C1-C4 alkyl group optionally sub-
stituted with a phenyl group, and R2 is hydrogen or a C1-C4
alkyl group.
[0107] The Derwent Abstract [Accession no. 1994-
107035] for Japanese patent application JP-A-6057218 dis-
closes a primer composition containing a tert-amine, where
the three groups are each a hydrocarbon group, but at least
one among them is a long chain hydrocarbon group con-
taining 8 to 24 carbon atoms. The active adhesion-promoting
agent is applied by dissolution in isopropanol. WO 91/ 18956
discloses imidazole derivatives such as 1-benzyl-2-meth-
ylimidazole, 1-benzylimidazole, 1-(2-thyl)-2-phenyl-4,5-di-
hydroimidazole, 2-phenyl-4,5-dihydroimidazole, and N,N‘-
carbonyldiimidazole, for use as primers in the bonding of
plastics, including polyolefins, with cyanoacrylate adhe-
s1ves.
[0108] U.S. Pat. No. 4,869,772 discloses di- and tri-
azabicyclo primer compositions for use in bonding non-
polar substrates. The active adhesion-promoting agents are
dissolved in a solvent, applied as a solution, and allowed to
dry leaving the adhesion-promoting agent on the substrate
before subsequent bonding with cyanoacrylate.
[0109] EP 0 333 448 (equivalent to U.S. Pat. No. 5,079,
098) discloses primer solutions (in low surface tension
US 2003/0164222 A1
organic solvents) of quaternary ammonium compounds of
the formula: R4N+ A‘ where R is alkyl, hydroxyalkyl, aryl,
alkaryl, aralkyl, and alkenyl, optionally substituted by het-
eroatoms; A‘ is an anion with pKa>0 in deprotonation
equilibrium reactions.
[0110] Anaerobic products with which certain primer
compositions (in particular cure-promoting primer compo-
nents) of the present invention are useful include polymer-
izable compositions which are often in the liquid state. The
(polymerizable) anaerobic product remains unpolymerized
in the presence of air but polymerizes upon exclusion of air.
This property of anaerobic products finds application as for
example a sealant between closely fitting metal surfaces.
Anaerobic product placed between closely fitting metal
surfaces such as flanges cures due to a lack of air.
[0111] Anaerobic products such as adhesives and sealant
compositions are known in the art. Examples of documents
which describe these products include U.S. Pat. Nos. 2,895,
950, 3,043,820, 3,218,305 (all to Krieble) and 4,287,330
(Rich) the entire contents of each of which is expressly
incorporated herein by reference.
[0112] In particular U.S. Pat. Nos. 2,895,950 and 3,043,
820 disclose anaerobic sealant compositions comprising
polymerisable compounds of the formula:
‘Ii i i ‘Ii
H2C:(|Z—C—O (CH2)m (I: (|:—o C—(|I:CH2
R’ R” H R’
P II
[0113] where R is selected from hydrogen, —CH3,
—C2H5, —CH2OH, and
0
||
—cH2—o—c—c=cH2
Ry
[0114] radicals, R‘ is selected from hydrogen, chlorine,
methyl and ethyl radicals; R“ is selected from hydrogen,
—OH radical, and
O
—o—c—c=cH2
Ry
[0115] radicals; m is an integer equal to at least one, e.g.
from 1 to 8 or higher, for instance from 1 to 4, inclusive, n
is an integer equal to at least 2, for example from 2 to 20 or
more, inclusive, and p is 0 or 1. Anaerobic compositions
including these polymerizable compounds are useful in
conjunction with the primer compositions of the present
invention in particular those primer compositions of the
present invention identified as being cure-promoting.
Sep. 4, 2003
[0116] U.S. Pat. No. 3,218,305 discloses anaerobic prod-
ucts containing polmerizable compounds of the formula:
‘Ii i i ‘Ii
H2c=c|:—c—o (CH2)m (I: (|:—o c—(|:=CH2
R’ R” P H R’
n
[0117] where R is a radical selected from hydrogen, C1-C 4
alkyl, or C1-C4 hydroxyalkyl or
O
—cH2—o—c—c=cH2
Ry
[0118] radicals, R‘ is selected from hydrogen, halogen, and
C1-C4 alkyl; and [text missing or illegible when filed],
m, n and p, are as defined above. Anaerobic compositions
including these polymerizable compounds are useful in
conjunction with the primer compositions of the present
invention in particular those primer compositions of the
present invention identified as being cure-promoting.
[0119] In particular polymerizable compounds useful in
the present invention may be:
‘Ii i i ‘Ii
H2c=c|:—c—o (CH2)m (I: (|:—o c—(|:=CH2
R’ R” H R’
P II
[0120] where R is a radical selected from hydrogen, C1-C4
alkyl, or C1-C4 hydroxyalkyl or
O
—cH2—o—c—c=cH2
Ry
[0121] radicals, R‘ is selected from hydrogen, halogen, and
C1-C4 alkyl, OH; and R“ is selected from hydrogen, —OH
radical, and
O
—o—c—(:=cH2
Ry
[0122] radicals; m is an integer equal to at least one, e.g.
from 1 to 8 or higher, for instance from 1 to 4, inclusive, n
is an integer equal to at least 2, for example from 2 to 20 or
more, inclusive, and p is 0 or 1.
US 2003/0164222 A1
[0123] These polymerisable compounds are typically for-
mulated in a composition which contains at least sufficient
components to make the composition curable. The person
skilled in the art will, depending on the polymerizable
monomer selected, consider any further appropriate compo-
nents. Typical further components for inclusion include an
initiator which may independently initiate or assist initiation
of polymerization in the absence of air. These initiators are
often oxidising agents. These initiators include peroxides for
example hydroperoxides such as cumene hydroperoxide.
[0124] A further component which may be added is a
reducing agent. Typical reducing agents include tertiary
amines and for instance U.S. Pat. No. 4,287,330 (Rich)
discloses rhodanine and organic hydrazines which are effec-
tive accelerators for the cure of anaerobic compositions.
Other suitable reducing agents are well known to those
skilled in the art. Another potential component is a co-
catalyst (for example saccharine) which may catalyse poly-
merization of the polymerizable compounds.
[0125] Suitable compositions for use with primer compo-
sitions of the present invention are described in detail in our
co-pending Irish application entitle “SEMI-SOLID ONE-
AND TWO-PART COMPOSITIONS” filed on even date
herewith in the name of Loctite (R&D) Limited.
[0126] The use of primer solutions to facilitate more rapid
cure, greater through depth cure, or improve adhesion to
substrates has been considered also for anaerobic products.
These primer solutions have been proposed in particular for
use on substrates which do not have readily leachable metal
ions or polar groups to facilitate effective adhesion. U.S. Pat.
Nos. 4,990,281 (Clark), 3,970,505 (Hauser), 3,591,438
(Toback), 3,625,930 (Toback), among others disclose the
use of various types of primers. The teaching of each of
these documents is expressly incorporated herein by refer-
ence. A common feature of all these primer composition is
that the active ingredient is contained in a solvent and the
primer solution has to be applied from the liquid state with
a variety of techniques.
[0127] The ’505 patent teaches the use of in particular a
substituted thiourea (in conjunction with an acid) as a cure
accelerator for anaerobic products. The substituted thiourea
(optionally together with the acid) can be used as a primer
to activate the surface to which it is desired to apply
anaerobic product. The substituted thiourea can be formu-
lated (optionally together with the acid) in a primer com-
position of the present invention.
[0128] The ’438 patent teaches the use of a reducing
activator which is either a sulphur-containing free radical
accelerator, or a compound containing an oxidizable transi-
tion metal. The compounds containing an oxidizable tran-
sition metal are especially useful in compositions of the
present invention and includes those compounds containing
the following transition metals: Cu, Cr, Co, Fe, and Mn.
Desirable compounds include salts and complexes of these
metals including mono- or poly-nuclear and homo- or het-
ero-nuclear compounds.
[0129] Other transition metal-based compounds are dis-
closed in the ’281 patent are desirable for inclusion. These
include Cu(II), Co(II), Mn(II), Mn(III) and Cr(II) based
compounds in particular salts of these metals. The ’281
patent is particularly concerned with the salts of these metals
Sep. 4, 2003
with acid phosphate acrylic monomer. Particular acid phos-
phate acrylic monomer disclosed include those of the for-
mula:
R10 0
cH2=c—c—R2—o—P—A
OH
[0130] where R1 is H or methyl, R2 is a divalent organic
group having from 2 to 20 carbon atoms and the group A is
OH or
R10
cH2=c—c—o—R2o—
[0131] where R1 and R2 are as defined for the formula
immediately above.
[0132] Suitable metal compounds include copper (I) and
(II) salts in particular those of carboxylic acids or those of
beta diketones or beta keto esters. Specific examples include
for example Cu(II) 2-ethylhexanoate and Cu(II) acetylac-
etonate.
[0133] A representative of a Cu(II) based primer is Loctite
product “Primer NTM”.
[0134] Other anaerobic products commercially available
from Loctite Corporation Rocky Hill Connecticut, US
include the following products sold under the following
trade names Loctite 636, Loctite 326, Loctite 648, Loctite
270, and Loctite 290.
[0135] The ’930 patent discloses a primer composition for
anaerobic adhesives which contains as accelerator a com-
pound having one of the following groups:
[0136] These compounds are also suitable for use with the
primer compositions of the present invention.
[0137] A container for holding a cast (solidified) compo-
sition of the invention is now described.
[0138] FIG. 1 shows a side view of a container 1 suitable
for holding a composition of the present invention. The
container is cylindrical in cross-section having cylindrical
side walls 2. On the base of the container is a knurled wheel
3 which forms part of a propulsion mechanism for a (soft-
solid or semi-solid) mass or stick 4 of the primer composi-
tion of the present invention. The mass 4 has been cast in a
generally cylindrical shape as described in the Examples
below. The container further comprises a cap 5 which is
snap-fit engageable over the top end 6 of the container 1 to
protect the mass 4 of product. The top end 6 is of lesser
diameter than the side walls 2 and has a rim 7 which engages
in a corresponding recess on the underside of the cap 5 to
secure the cap 5 in place.
US 2003/0164222 A1
[0139] The knurled wheel 3 is attached to an elongate
drive or winding shaft 8 which is centrally located within the
housing formed by the side walls of the container. On the
winding shaft 8 is located a moveable carrier 9. The carrier
9 is generally cylindrical (from an end View thereof—see for
example FIG. 2) and has a short peripheral upstanding wall
10 formed on its base 11. During the casting process the
carrier 9 is positioned to secure itself to the lower end 12 of
the mass 4 on solidification of the mass 4. Indeed the mass
4 may be cast also with the shaft 8 (and optionally the wheel
3) in place. As best seen from FIG. 2 the carrier 9 has a
central threaded aperture 13 in which the threads 16 of the
shaft 8 engage. The knurled wheel 3 and the shaft 8 are both
[text missing or illegible when filed] for relative rota-
tion to the container body. When the wheel 3 is turned in the
direction of the arrow it moves the carrier up or down the
shaft 8 thus controlling the relative position of the mass and
the container. In the position shown the carrier has travelled
part way up the shaft, moving the mass to a position where
it protrudes from the container. The mass can then be applied
by rubbing against a substrate by manual force. Sufficient
shearing of the mass takes place to allow it to rub off onto
the substrate. No separate applicator/brush etc. is necessary.
The composition can be applied with manual pressure. To
prevent rotation of the carrier 9 with the shaft, elongate ribs
14 are provided on opposing sides of the internal wall of the
container. The ribs 14 run from the base of the container to
a position proximate to the mouth if the container. The ribs
14 each engage one of corresponding grooves 15 in the
carrier 9 thus preventing relative rotation of the container
and the carrier and ensuring that the carrier moves upwardly
or downwardly when the shaft 8 turns. The carrier 9 and the
mass 4 can be retracted by rotation of the wheel 3 in an
opposing direction.
[0140] The words “comprises/comprising” and the words
“having/including” when used herein with reference to the
present invention are used to specify the presence of stated
features, integers, steps or components but does not preclude
the presence or addition of one or more other features,
integers, steps, components or groups thereof.
[0141] The following examples will serve to illustrate the
invention.
EXAMPLES
[0142] General
[0143] In the following examples the dibenzylidene sor-
bitol used was the product DisorbeneTM described above.
[0144] Bonding tests were carried out using various sub-
strates, including natural polyethylene and natural polypro-
pylene as the non-polar substrates, various solutions of the
active adhesion promotion compounds as the primer and
various grades of cyanoacrylate adhesive commercially
available from Loctite (Ireland) Limited, Dublin, Ireland.
The bond strength of the resulting joints was determined
using conventional [text missing or illegible when
filed] following standard test method ASTM D 1002.
[0145] The 3 kg Test as described below is the minimum
time following assembly for bonds to support a 3 kg weight.
The minimum time is determined in accordance with ASTM
D1002.
Example 1
[0146] 2.0 g of dibenzylidene sorbitol was dissolved in
refluxing ethanol (148 g) with vigorous stirring and the
Sep. 4, 2003
solution allowed to cool for a short period. Then 1,8-
diazabicyclo[5.4.0]undec-7-ene (0.15 g) was added with
stirring and the solution was allowed to cool further. The
solution was then cast into empty stick cartridges of the type
typically used for adhesives such as PrittStickTM (and as
described above) and was allowed to cool to room tempera-
ture. During this time the solution solidified. The solidified
stick was clear (homogeneous) resembling a frozen mass.
The solid stick primer solution could then be extruded using
the cartridges inbuilt propulsion mechanism.
[0147] Test pieces of natural polyethylene of dimensions
100 mm>