2-Cyanoacrylate Adhesive Composition
Folder:
Year:
Abstract:
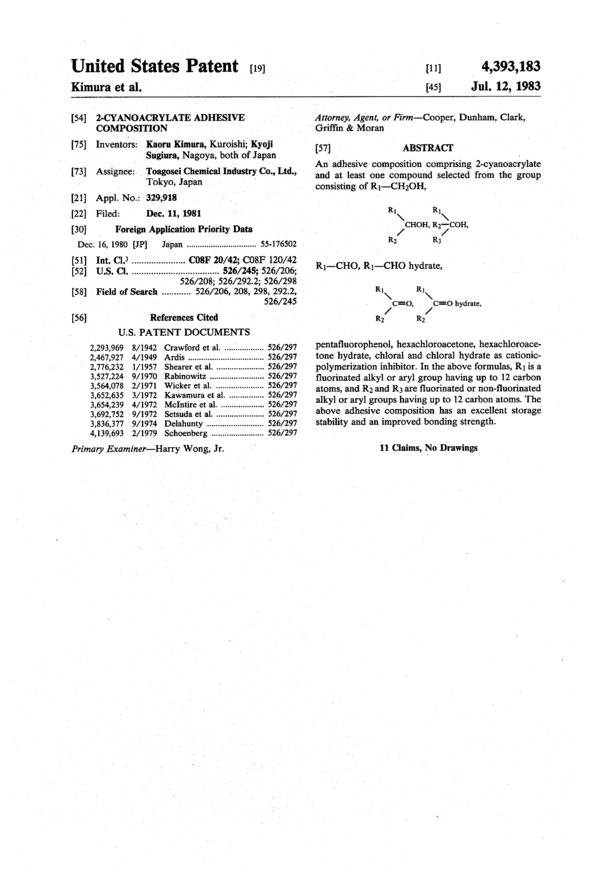
An adhesive composition comprising 2-cyanoacrylate and at least one compound selected from the group consisting of R1-CH2OH,
pentafluorophenol, hexachloroacetone, hexachloroacetone hydrate, chloral and chloral hydrate as cationic-polymerization inhibitor. In the above formulas, R1 is a fluorinated alkyl or aryl group having up to 12 carbon atoms, and R2 and R3 are fluorinated or non-fluorinated alkyl or aryl groups having up to 12 carbon atoms. The above adhesive composition has an excellent storage stability and an improved bonding strength.
Type of document:
Language:
_[22] Filed:
United States Patent [19]
Kimura et al.
[54] 2-CYANOACRYLATE ADHESIVE
COMPOSITION ’
[75] Inventors: Kaoru Kimura, Kuroishi; Kyoji
Sugiura, Nagoya, both of Japan
Toagosei Chemical Industry Co., Ltd.,
Tokyo, Japan
[21] Appl. No.: 329,918
Dec. 11, 1981
[30] Foreign Application Priority Data
Dec. 16, 1980 [JP] Japan .............................. .. 55-176502
[51] Int. Cl.3 .................... .. C08F 20/42; COSF 120/42
[52] U.S. Cl. .................................. .. 526/245; 526/ 206;
526/208; 526/292.2; 526/298
[58] Field of Search .......... .. 526/206, 208, 298, 292.2,
526/245
[73] Assignee:
[56] References Cited
U.S. PATENT DOCUMENTS
2,293,969 8/1942 Crawford et al. ................ .. 526/297
2,467,927 4/1949 Ardis .............. .. 526/297
2,776,232 1/1957 Shearer et al. 526/297
3,527,224 9/1970 Rabinowitz .. 526/297
3,564,078 2/1971 Wicker et al. 526/297
3,652,635 3/1972 Kawamura et a1. . 526/297
3,654,239 4/1972 Mclntire et al. 526/297
3,692,752 9/1972 Setsuda et al. 526/297
3,836,377 9/1974 Delahunty‘ ..... .. 526/297
4,139,693 2/1979 Schoenberg ...................... .. 526/297
Primary Examiner—Harry Wong, Jr.
[11] 4,393,183
[45] Jul. 12, 1983
Attorney, Agent, or Firm—Cooper, Dunham, Clark,
Griffin & Moran
[57] ABSTRACT
An adhesive composition comprising 2-cyanoacrylate
and at least one compound selected from the group
consisting of R1—CH2OH,
R1 R1
\ \
CHOH, R2-—C0H,
/
R2 R3
R1-4-CH0, R1———CHO hydrate,
R1 R1
\ \
C=O,
C=O hydrate,
/
R2 R2
pentafluorophenol, hexachloroacetone, hexachloroace-
tone hydrate, chloral and chloral hydrate as cationic-
polymerization inhibitor. In the above formulas, R1 is a
fluorinated alkyl or aryl group having up to 12 carbon
atoms, and R2 and R3 are fluorinated or non-fluorinated
alkyl or aryl groups having up to 12 carbon atoms. The
above adhesive composition has an excellent storage
stability and an improved bonding strength.
11 Claims, 1_‘Io Drawings
4,393,183
1
2-CYANOACRYLATE ADHESIVE COMPOSITION
The present invention relates to a 2-cyano acrylate
adhesive composition. More particularly, it relates to a
2-cyanoacrylate adhesive composition having an excel-
lent storage stability and an improved bonding strength.
2-Cyanoacrylate adhesives are reactive adhesives
which comprise 2-cyanoacrylate monomer as the main
component, and therefore, they are often cured during
storage in their containers owing to the radical or ani-
onic polymerization of 2-cyanoacrylate monomer. In
order to obtain a good storage stability, a polymeriza-
tion inhibitor is added to them as a stabilizer. As inhibi-
tors for the radical polymerization of 2-cyanoacrylate
monomer, there are used hydroquinone, hydroquinone
monomethyl ether, catechol, pyrogallol, Bisphenol A
and the like. The inhibitor is added in the range of 1 to
10,000 ppm in order to prevent the radical polymeriza-
tion during storage at room temperature.
On the other hand, the anionic polymerization is
initiated by a very small amount of a basic substance
such as water, an amine, ammonia or the like incorpo-
rated during storage. For preventing the viscosity in-
crease and gelation resulting from this anionic polymer-
ization, it is known that S02, S03, SOCI2, SO2Cl2, HF,
N02, p-toluenesulfonic acid, methanesulfonic acid, pro-
panesultone, phosphoric acid, sulfuric acid and the like
are effective. However, the addition of these anionic-
polymerization inhibitors causes the coloring of adhe-
sive composition with time and the prolongation of the
setting time thereof with time. Accordingly, anionic-
polymerization inhibitors of better quality which do not
cause the above problems have been desired in this field.
In view of the above need, the present inventors have
made research on stabilizers which can prevent the
anionic polymerization of 2-cyanoacrylate monomer.
As a result, it has been found that a compound having
an active hydroxyl group or a group which can be
converted into an active hydroxyl group is an effective
anionic-polymerization inhibitor unlike the conven-
tional one. ‘
According to this invention, there is provided an
adhesive composition which comprises a 2-cyanoacry-
late and a compound having an active hydroxyl group
or a group which can be converted into an active hy-
droxyl group. The present invention has enabled the
prevention of the reduction in the adhesive properties
of 2-cyanoacrylate monomer with time during storage
experienced with conventional anionic-polymerization
inhibitors, namely the prevention of the coloring with
time and the prolongation of setting time with time.
Further, the adhesive composition according to the
present invention has not only excellent storage stabil-
ity, but also an improved bonding strength.
The present anionic-polymerization inhibitor for 2-
cyanoacrylate monomer which has an active hydroxyl
group or a group which can be converted into an active
hydroxyl group is a compound possessing in the mole-
cule fluorine or chlorine atoms and a hydroxyl, alde-
hyde or carbonyl group. Specifically, the following
compounds are included:
10
15
20
25
30
35
40
45
50
55
65
2
R1 R1 (1)
\ \
R1‘-CH7_OH, CHOH, R2“COH;
/ /
R2 R3
(2) R1—CHO, R1—CHO hydrate;
(3)
C=O hydrate;
R2
(4) pentafluorophenol;
(5) hexachloroacetone, hexachloroacetone hydrate; and
(6) chloral, chloral hydrate.
In the above formulas, R1 is a fluorinated alkyl or aryl
group having up to 12 carbon atoms; and R2 and R3 are
fluorinated or non-fluorinated alkyl or aryl groups hav-
ing up to 12 carbon atoms.
Among the above compounds, the compounds (1),
(2), (3), (4) and (5) are preferred, and more preferable
are the compounds represented by the formulas,
R] R1 R1
\ \ \
R2—COI-I, C=O, C=O hydrate,
/ / /
R3 R2 R2
and pentafluorophenol.
Specific examples of these compounds include triflu-
oroethanol, 1H,1H-pentafluoropropanol, 1H,lH-hepta-
fluorobutanol, lH,1H-nonafluoropentanol, 1, l, 1-tri-
fluoroisopropanol, hexafluoroisopropanol, octafluoro-
sec-butanol, perfluoro-tert-butanol, hexafluoro-tert-
butanol, 2-trifluoromethylpropanol-2, 1-ch1oro-
1,1,3,3,3-pentafluoro-2-propanol, 3,3,4,4,4-penta-
fluorobutanol-2, 3,3,4,5,5,5-hexafluoro-2-methylpen-
tanol-2, 1H,1H,5H-octafluoro-1-pentanol, 1H, lH-pen-
tadecafluorooctanol-1, trifluoroacetaldehyde, tri-
fluoroacetaldehyde hydrate, heptafluorobenzaldehyde,
2,2,3,3,4,4-hexafluoro-1,5-pentanediol, pentafluoroben-
zyl alcohol, hexafluoroacetone, hexafluoroacetone hy-
drate, trifluoromethyl trichloromethyl ketone, penta-
fluoroethyl ethyl ketone, pentafluorophenyl methyl
ketone, methyl heptafluoropropyl ketone, hexafluoro-2-
phenylisopropanol, hexafluoro-2-(p-tolyl)isopropanol,
pentafluorophenol, hexachloroacetone, hexachloroace-
tone hydrate, sym-dichlorotetrafluoroacetone and sym-
dichlorotetrafluoroacetone hydrate.
Among the above specific compounds, preferable are
trifluoroethanol, 1H,1H—pentafluoropropanol, 1, 1, l-tri-
fluoroisopropanol, hexafluoroisopropanol, octafluoro-
sec-butanol, perfluoro-tert-butanol, hexafluoro-tert-
butanol, 2-trifluoromethylpropanol-2, 3,3,4,4,4-penta-
fluorobutanol-2, trifluoroacetaldehyde, tri-
fluoroacetaldehyde hydrate, heptafluorobenzaldehyde,
pentafluorobenzyl alcohol, hexafluoroacetone, hexa-
fluoroacetone hydrate, trifluoromethyl trichloromethyl
ketone, pentafluorophenyl methyl ketone, methyl hep-
tafluoropropyl ketone, pentafluorophenol, hexachlor-
oacetone, and sym-dichlorotetrafluoroacetone. More
preferable are hexafluoroisopropanol, perfluoro-tert-
butanol, trifluoroacetaldehyde, trifluoroacetaldehyde
hydrate, hexafluoroacetone, hexafluoroacetone hydrate
and pentafluorophenol.
4,393,183
3
These compounds possess a hydroxyl group activated
by electron-attractive fluorine or chlorine atoms, or, in
the case of aldehyde and ketone compounds, they can
be converted into compounds having active hydroxyl
group as shown in the following formulas, by combin-
ing or reacting with a very small amount of water or an
alcohol present in the adhesive composition:
OH
H 0 /
CF3CHO 2% CF3CH
OI-I
c H OH
CF3CHO -—2—5—-9 CF3(|2H--OH
OCZH5
/on
H 0
CF3CCH3 2% (CF3)2C
ll
0 OH
The above fluorinated or chlorinated compounds
have the nature that they become acidic because of the
dissociation of hydroxyl groups into H+ due to the
electron attractive property of the fluorinated or chlori-
nated alkyl or aryl groups. Therefore, these compounds
prevent the anionic polymerization of 2-cyanoacrylate
monomer during storage, and accordingly, no reduc-
tion of its bonding performance with time takes place.
The 2-cyanoacrylate adhesive composition according
to the present invention can be obtained by adding at
least one compound selected from the above com-
pounds (1) to (6) to a 2-cyanoacrylate monomer, prefer-
ably in a quantity of 0.1 to 10,000 ppm, more preferably
1 to 6,000 ppm. In this case, the above compound may
also be used in combination with a conventional anion-
ic-polymerization inhibitor such as S02, S03, p-toluene-
sulfonic acid, propanesultone or the like.
Representative examples of the 2-cyanoacrylate mon-
omer, which is the main component of the adhesive
composition according to the present invention, include
methyl 2-cyanoacrylate, ethyl 2-cyanoacrylate, propyl
2-cyanoacrylate, allyl 2-cyanoacrylate, butyl 2-cyanoa-
crylate, heptyl 2-cyanoacrylate, hexyl 2-cyanoacrylate,
octyl 2-cyanoacrylate, decyl 2-cyanoacrylate, dodecyl
2-cyanoacrylate, 2-chloroethyl 2-cyanoacrylate, methyl
Cellosolve 2-cyanoacrylate, ethyl Cellosolve 2-cyanoa-
crylate, butyl Cellosolve 2-cyanoacrylate, benzyl 2-
cyanoacrylate, phenyl 2-cyanoacrylate, trifluoroisopro-
pyl 2-cyanoacrylate, and the like.
The adhesive composition according to the present
invention may also contain a radical polymerization
inhibitor in a quantity of l to 10,000 ppm, preferably 10
to 5,000 ppm. For example, hydroquinone, hydroqui-
none monomethyl ether, catechol and pyrogallol are
effective.
When it is desired to thicken the present adhesive
composition, a thickener such as methyl methacrylate
polymer, vinyl acetate polymer, cellulose acetate isobu-
tyrate, acrylic rubber or the like may also be dissolved
in a proportion of several percent.
Other additives such as plasticizers for imparting
flexibility to cured adhesive layers, modifiers for im-
parting impact resistance and heat resistance and dyes
and pigments for identification of coating may also be
added.
Because of containing a small amount of at least one
of the compounds (1) to (6), the adhesive composition
l0
15
20
25
30
35
45
50
55
60
65
4
according to this invention exhibits aniexcellent storage
stability and simultaneously has a higher bonding
strength than the conventional adhesive compositions.
The adhesive composition according to this invention
has a high setting rate (namely a short setting time) and
is suitable for instantaneous adhesion of most materials
such as rubbers, plastics, metals, wood and the like. The
composition is used, for instance, for assembling small
parts.
This invention is explained in more detail below re-
ferring to Examples. However, this invention should
not be understood to be limited to the Examples. In the
Examples and Comparative Examples, parts are by
weight unless otherwise specified.
Examples 1 to 17 and Comparative Example 1
To 100 parts of ethyl 2-cyanoacrylate were added
0.05 part of hydroquinone and 0.002 part of each of the
inhibitors shown in Table 1. Each of the adhesive com-
positions thus obtained was charged into a polyethylene
container and then subjected to heating tests at 60° C.
Results are shown in Table 1.
TABLE 1
Tensile
shear
strength
of bond
(iron)
(Kgf/cmz)
Setting
time
(iron)
(sec)
Heat
gelation
test at
Inhibitor 60° C.
Ex-
ample
1 CF3Cl-I201-I 10 200 Stable for
more than
35 days
Stable for
more than
35 days
Stable for
more than
46 days
2 (CF3)2CHOH 10 205
3 (CF3)3COH 10 205
4 CH3 10 200 Stable for
I more than
CF3"CHOH 35 days
5 CF3CF2Cl-I201-I 10 200 Stable for
more than
35 days
Stable for
more than
35 days
Stable for
more than
43 days
Stable for
more than
35 days
Stable for
more than
50 days
Stable for
more than
35 days
Stable for
more than
40 days
Stable for
more than
35 days
Stable for
more than
43 days
Stable for
more than
45 days
6 HCFZCFZCI-I201-I 10 210'
7 C5F5Ol-I 10 210
3 C5F5CH20H 10 220
9 (CF3)2C=O hydrate 10 220
10 (cc13-)2—c=o 10 200
ll CF3CHO hydrate 10 210
12 10
CCl3CI-IO 190
13 C5F5CHO 10 195
14 (cc11=2-)z—c=o
hydrate
10 200
4,393,183
5 6
TABLE 1-continued TABLE 3
Tensile Tensile
shear shear
Setting strength Heat Strength Heat‘
time ‘ of bond gelation 5 al Seniflg 0f bond 8913*)“
(iron) (1,-oh) test gt fsddition time (iron) test at
Inhibitor (sec) (Kg,-/cmz) 60- C_ Inhibitor (sec) (Kgf/cmz) 60' C.
is C3F7CCH3 10 200 Stable for _1:3_X2I2L1
Coments go here:
- Log in to post comments