52
WEISSBERGERN D H. D.
4
[COMMUNICATION NO.887 FROM THE
PORTER
Vol. 65
KODAK
RESEARCH
LABORATORIES]
Xnvestigation of Pyrazole Compounds. 1.
1' The Synthesis of
hydroxy-5-pyrazolone Imide
l-Phenyl-3-
BY A. WEISSBERGER H. D. PORTER
AND
I n the first paper of this series i t was shown that samples, and the color test with p-aminodimethylthe compound synthesized by Conrad and Zart? aniline, confirmed their identity.
I1 differs from I by its higher solubility in all
and called 1-phenyl-3-hydroxy-5-pyrazolone
imide
is in fact the isomeric l-phenyl-3-amino-5-pyrazo-solvents. While I is almost insoluble in water,
lone I. The real l-phenyl-3-hydroxy-5-pyrazolone I1 can be recrystallized from i t t o give two polyimide I1 has now been prepared. Phenylhydra- morphic forms. The crystals which separate
zine was condensed with cyanoacetyl chloride to rapidly from a concentrated (7 ml./g.) solution
give 0-cyanoacetylphenylhydrazine 111. In- melt a t 14Z0, and those which separate slowly from
stead of the acid chloride, cyanoacetazide3 can be a more dilute (20 ml./g.) solution a t 160'. The
used, giving a somewhat higher yield. I11 is a melt of either form on cooling t o room temperacolorless well-crystallizing substance. It is sol- ture yields the lower-melting polymorphic variety.
uble in aqueous sodium carbonate, slightly sol- However, if the melt is kept a t a temperature
uble in cold water, and the addition of mineral only a few degrees below the lower melting point,
acid does not increase its solubility in the latter. the high-melting form is obtained.
With acetic anhydride or benzoyl chloride, even
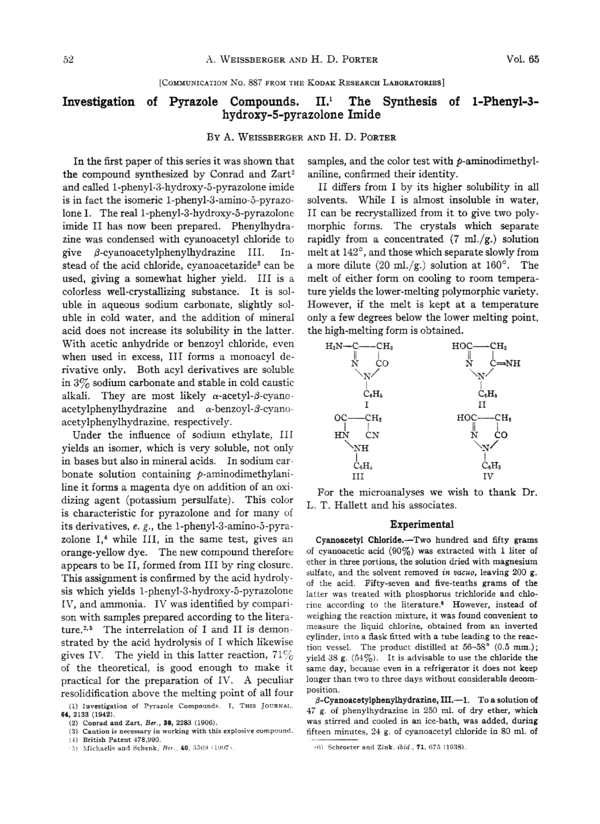
H~N-C-CHZ
HOC-CHI
II
I
/I
I
when used in excess, 111 forms a monoacyl deN
C=NH
N
CO
rivative only. Both acyl derivatives are soluble
*\N/
\N/
in 3% sodium carbonate and stable in cold caustic
I
I
,
CEH~
CaHs
alkali. They are most likely a-acetyl-@-cyanoI
I1
acetylphenylhydrazine and a-benzoyl-b-cyanoHOC-CHL
OC-CH2
acetylphenylhydrazine, respectively.
II
I
I
I
Under the influence of sodium ethylate, 111
HN
CN
N
CO
yields an isomer, which is very soluble, not only
\NH
\N/
I
I
in bases but also in mineral acids. In sodium carCC"
C6Ha
bonate solution containing p-aminodimethylaniI11
IV
line i t forms a magenta dye on addition of an oxiFor the microanalyses we wish to thank Dr.
dizing agent (potassium persulfate). This color L. T. Hallett and his associates.
is characteristic for pyrazolone and for many of
Experimental
its derivatives, e. g., the 1-phenyl-3-amino-5-pyrazolone I,4 while 111, in the same test, gives an
Cyanoacetyl Chloride.-Two hundred and fifty grams
orange-yellow dye. The new compound therefore of cyauoacetic acid (90%) was extracted with 1 liter of
appears to be 11, formed from 1 1by ring closure. ether in three portions, the solution dried with magnesium
1
sulfate, and the solvent removed in vacuo, leaving 200 g.
This assignment is confirmed by the acid hydroly- of the acid. Fifty-seven and five-tenths grams of the
sis which yields l-phenyl-3-hydroxy-5-pyrazolonelatter was treated with phosphorus trichloride and chloIV, and ammonia. I V was identified by compari- rine according to the 1iterature.O However, instead of
son with samples prepared according to the litera- weighing the reaction mixture, it was found convenient to
t ~ r e . ~ The interrelation of I and I1 is demon- measure the liquid chlorine, obtained from an inverted
J
cylinder, into a flask fitted with a tube leading to the reacstrated by the acid hydrolysis of I which likewise tion vessel. The product distilled a t 56-58" (0.5 mm.);
gives IV. The yield in this latter reaction, 717; yield 38 g. (54%). I t is advisable to use the chloride the
of the theoretical, is good enough t o make it same day, because even in a refrigerator it does not keep
practical for the preparation of IV. A peculiar longer than two to three days without considerable decomresolidification above the melting point of all four position.
(1) Investigation o Pyrazole Compounds. I , THIS O U R N A L .
f
J
64, 2133 (1942).
(2) Conrad a n d Z a r t , Bcr., 39, 2283 (1906).
(3) Caution is necessary i n working with this explosive compound.
$1 British P a t e n t 478,990.
3 ) Alichaeli.; a n d Schenk, Her 40, 33!jR l1!407!
B-Cyanoacetylphenylhydrazine,
111.-1.
To a solution of
47 g. of phenylhydrazine in 250 ml. of dry ether, which
was stirred and cooled in an ice-bath, was added, during
fifteen minutes, 24 g. of cyanoacetyl chloride in 80 ml. of
I!;)
Schroeter and 'Link. ibid , 71, 675 (1938)
�Jan., 1943
THESYNTHESIS OF l-PHENYL-3-HYDROXY-5-PYRAZOLONE
IMIDE
dry ether. Stirring and cooling was continued for one
hour, the granular crystalline mass collected at the pump,
rinsed with ether, and slurried and filtered twice with 400
ml. of water. The solid was extracted with 150 ml. of
boiling 60% ethanol, leaving 7 g. of high melting insoluble
residue. The solution on cooling gave 15.5 g. of creamcolored needles, m. p. 98-101', and recrystallized (Norite)
twice from 50% ethanol, 10 g. (33%) of fine white needles;
m. p. 105-106'.
Anal. Calcd. for CQHQN~O: 61.7; H, 5.14; N,
C,
24.0. Found: C, 61.59; H, 4.88; N, 24.00.
2. A suspension of 84 g. of cyanoacethydrazide7 in 300
ml. of water and 300 ml. of ethyl ether was cooled to 5' and
71 ml. of concd. hydrochloric acid was added with stirring.
After cooling to O', 58 g. of sodium nitrite in 150 ml. of
water was added within ten to fifteen minutes, while stirring vigorously, a t a reaction temperature maintained below 10" by the addition of dry-ice.
After stirring and cooling for another fifteen minutes,
90 g. of phenylhydrazine was added dropwise during fifteen
minutes at 5-10'. After another hour, the mixture was filtered. The residue was slurried with 300 ml. of ether, filtered, rinsed with ether, then washed with 150 ml. of water,
and recrystallized from 350 ml. of 35% ethanol, 75 g
(52%) of fine white needles; m. p. 105-106'.
The isolation of the cyanoacetazide,T after the fmt step
in the above reaction, is dangerous. This isolation was
done with several samples until a small batch, after one
day's standing, detonated with extreme violence when the
ethereal solution was concentrated. Moreover, it was
found that a better over-all yield was obtained with the
procedure given above than when the azide was isolated.
If, in the preparation of the azide, the nitrite solution is
added more slowly, a solid (up to 16%) separates from the
reaction mixture. The compound crystallized from water
in thick needles, m. p. 194-196', and is presumably a,@-di(cyanoacetyl)-hydrazine.
Anal. Calcd. for CsHsNdOz: C, 43.4; H. 3.61; N,
33.7. Found: C, 43.69; H, 3.89; N, 33.56.
Coupling Test with p-Aminodimethylanie.-A
small
amount (about 0.01 g.) of 8-cyanoacetylphenylhydrazine
was dissolved in 5 ml. of 3% aqueous sodium carbonate
containing about 0.01 g. of p-aminodimethylanie, and
to the solution was added about 2 ml. of 2% potassium
persulfate solution. Immediately, a bright yellow-orange
color appeared which faded on addition of mineral acid.
a-Acetyl-P-cyanoacetylphenylhydrazine.-One gram of
b-cyanoacetylphenylhydrazine 5 ml. of acetic anhydride
in
was heated on the steam-bath for one hour. The solution
was vacuum-concentrated, and the residue taken up in 5
ml. of hot benzene from which crystals separated. These
were recrystallized twice from methanol, yielding fine
white needles; m. p. 149-150'.
Anal. Calcd for CIIHIIN~OZ: 19.35. Found: N,
N,
19 27.
a-Benzoyl-8-cyanoacetylphenylhydrazine.-To a solution of 1.75 g. of 6-cyanoacetylphenylhydrazine and 1.6 g.
of pyridine in 3.5 ml. of dioxane, was added 2.8 g. of benzoyl
chloride. After heating on the steam-bath for half an
hour, excess benzoyl chloride was decomposed by adding
(7) Darapsky and Hillers J p r a k f Chem , Sa, 297 (1915)
53
5 m . of methanol, and the mixture poured into water.
l
The precipitated o l was washed with water and crystali
lized from benzene, yielding 1.2 g. (43%) of white needles;
m. p. 153-155'; recrystallization from methanol raised
the m. p. to 155-156'.
Anal. Calcd. for CIBHIIIN~OZ: 15.05. Found: N,
N,
15.12.
Both acyl derivatives were recovered unchanged on
acidification after standing for one hour in 2% sodium
hydroxide solution.
l-Phenyl-3-hydroxy-5-pyrazolone Imide, 11.-A solution of 80 g. of p-cyanoacetylphenylhydrazine in sodium
methylate (21 g. of sodium in 320 ml. of methanol) was
refluxed for one hour. It was then concentrated in uucuo
to dryness and the residue dissolved in 400 ml. of water.
On acidifying with 60 ml. of glacial acetic acid, heating,
Noriting, cooling, and filtering, 70.5 g. of crude product was
obtained which half melted at about 140', and totally a t
158'. Recrystallized from 500 ml. of water, it formed fine
white needles, m. p. 142-143'; yield, including 7.5 g. from
the filtrate, 59.5 g. (74%). A polymorphous form of m. p.
160.5-161.5' was obtained as described on page 52.
Anal. Calcd. for CeHsNaO: C. 61.7; H, 5.14; N, 24.0.
Found: (142') C, 61.81; H, 4.96; N, 24.01. Found:
(160') C, 61.55; H, 5.36; N, 23.8.
l-Phenyl-3-hydroxy-5-pyrazolone
imide was also formed
when a solution of 6-cyanoacetylphenylhydrazine in 2%
sodium hydroxide stood at room temperature for one hour.
However, under these conditions, the solution darkened
considerably and the yield was not as good as in the above
procedure.
In the coupling test (carried out by the method described
above), I1 formed a magenta dye which faded on addition
of mineral acid or of caustic alkali.
l-Phenyl-3-hydroxyy-5-pyrazolone,
IV.-1.
A suspension of 150 g. of l-phenyl-3-amino-5-pyrazolone~a mixin
ture of 3 liters of water, 450 ml. of 95% ethanol, and 110
ml. of concd. hydrochloric acid was stirred on the steambath. As soon as solution was complete (fifteen minutes),
it was Norited and filtered, heating the filtrate for fortyfive minutes longer. After cooling, the crystals were collected a t the pump and washed with water to give 107.5 g.
(710/o) of cream-colored plates; m. p. on rapid heating
(5' per min.) 193-195' dec. The melt reset to a semi-solid
a t 205'.
2. A solution of 0.25 g. of l-phenyl-3-hydroxy-5pyrazolone imide in 5 ml. of water and 0.25 ml. of concd.
hydrochloric acid was heated on the steam-bath for one
hour. On cooling, 0.05 g. of white needles crystallized
out. The yield was not increased by extending the time of
heating to three hours, probably because IV itself is destroyed by hydrolysis. The product was recrystallized
from water t o give fine white plates; m. p. on rapid heating
193-195' dec. The melt reset to a semi-solid a t 205'.
Mixed melting points of both preparatioq with each other
and with samples of l-phenyl-3-hydroxy-5-pyrazolone,
prepared according to the literature,*Vsshowed no depression.
I and I1 were recovered unchanged on acidification, after
heating their solutions in 2% sodium hydroxide for one
hour on the steam-bath.
�HERBERT s. HARNED
AND CLAIR M. BIRDSALL
54
Vol. 65
summary
razolone imide and l-phenyl-3-arnino-5-pyrazolone is shown by acid hydrolysis of both com1. 1- Phenyl - 3 - hydroxy - 5 - pyrazolone imide
was prepared by ring closure of @-cyano- pounds to l-PhenYl-3-hYdroxY-5-PYraZOlOne.
acetylphenylhydrazine obtained from phenyl3. Color reactions of p-cYanoacetYlPhenYlhYhydrazine and cyanoacetyl chloride or cyano- drazine and of the pyrazolone derivatives are deacetazide.
scribed.
2. The relation of 1-phenyl-3-hydr0xy-j-p~ROCHESTER, YORK RECEIVED
NEW
OCTOBER 14,1942
[CONTRIBUTION
FROM
THE
DEPARTMENT CHEMISTRY YALEUNIVERSITY]
OF
OF
The Acidic Ionization Constant of Glycine in Dioxane-Water Solutions
BY HERBERT
S. HARNED CLAIRM. BIRDSALL'
AND
The ionization constants of acetic, formic, propionic acids and water2 have been determined
from 0 to 50' in water and in dioxane-water
solutions from cells without liquid junction. In
order t o extend these results to include an ionization of another type of weak electrolyte, cells of
the type
H&* ( m A ,
HZCl ( m d , X%D, Y%H20IAgCl-Ag
have been employed to evaluate the acidic ionization constant of glycine as a function of the
composition of a medium of varying dielectric
constant and of the temperature. In this cell,
2" represents the amphion, +NHaCHsCOO-,
HZCl, glycine hydrochloride and X the percentage by weight of dioxane in the solvent. From
these ionization data, the entropy, heat content
and heat capacity of the ionization reaction may
be evaluated with a fair degree of accuracy.
Cells of this type have been employed frequently3 in recent years to determine the acidic
ionization constant of amino acids in water, and
without modification may be adapted to the investigation of ionization equilibrium in waterorganic solvent mixtures. The ionization under
consideration is given by the expression
"HZ
Z*
+ Hi'
and the corresponding equation for the thermodynamic ionization constant is
(1) This contribution contains material from a dissertation presented by Clair M. Birdsall t o the Graduate School of Yale University in partial fulfillment of the requirements for the degree of
Doctor of Philosophy, June, 1942.
(2) Harned and Kazanjian, THIS JOURNAL,
68, 1912 (1936);
Hnrned and Fallon, ibid., 61,2374 (1939); Harned and Done,ibid.,
63,2579 (1941); Harnedand Dedell, i b i d . , 63,3308 (1941).
(3) Harned and Owen, ibid., 62, 5091 (1930); Harned and Owen,
JOURNAL,
66,24(1934); Nims
Chem. Rev., fl, 31 (1939); Owen, THIS
101,401 (1933); P. K.
Smith, A . C.Taylor
and Smith, J. Biol. Chen..
and E . R . R S m i t h , ibid., 12.2, 109 (1937).
where m represents molality, y activity coefficient
and the ionic species are designated by the subscripts, 2, ZH and H.
The thermodynamic equation for the cell is
E = Eo*
- R T / N F In Y H Y c ~mHmCl
(2)
where Eo* is the standard potential in a given
solvent. Since YHYCl in a solution containing
glycine is not exactly known but can only be
approximated by employing values for hydrochloric acid a t the appropriate ionic strength in a
solvent which does not contain glycine, i t is necessary to define the quantities M H ' and KA' by the
equations
(4)
since mCl = m = mHZ. As the ionic strength
2
decreases, the apparent hydrogen ion concentration m H ' approaches the actual hydrogen ion
concentration mH so that at infinite dilution
KA' equals KA. Eo* and YHCl have been deter2
mined by Harned and Morrison,4 m is known, so
that measurement of E yields all the data necessary for the computation of m H ' . From these
values of mH' determined a t a number of suitable
concentrations, KA' is determined by equation
(3) and extrapolated to zero ionic strength where
it equals the thermodynamic ionization constant
KA *
Experimental Procedure and Observed Electromotive Forces.-The experimental technique
described in detail by Harned and Morrison5
(4) Harned and M o m s o n , THISJOURNAL, 1908 (1936).
68,
(5) Harned and M o m s o n , A m . J . Sci., 33,161 (1937)
�