New Pyrazolo-Pyrimidines
Folder:
Year:
Abstract:
This invention relates to a new pyrazolo-pyrimidines and a process for their preparation. More particularly the invention concerns hydro-pyrazolo (3,4-d) pyrimidines having the nucleus of the formula
-
which contain at least one of the positions 4 and 6 an oxo group and are alkylated, oxyalkylated, e.g. oxyethylated or cycloalkylated at at leats one ring nitrogen atom, their tautomeric forms and salt thereof.
Type of document:
Language:
United States Patent ‘Office-
3 ,098,075
Patented July 16, 1963
1
. 3,098,075 ’ ’
NEW PYRAZOLO-PYRIMIDINES
Jean Druey, Riehen, Paul Schmidt, Therwil, and Kurt
Eichenberger, Basel, Switzerland, assignors to Ciba Cor-
poration, a corporation of Delaware
No Drawing. Filed May 26, 1959, Ser. No. 815,825
Claims priority, application Switzerland Feb. 10, 1956
’ 24 Claims. (Cl. 260-—256.4)
This invention relates to new pyrazolo-pyrimidines and
a process for their preparation. , More particularly, the
invention concerns hydro-pyrazolo(3,4-d)pyrimidines
having the nucleus of the formula
4
N5
6 2N
\§, \§;/
which contain in at least oneof the positions 4 and 6 an
oxo group and are alkylated, oxyalkylated, e.g. oxyeth-
ylated or cycloalkylated at at least one ring nitrogen atom,
their tautomeric forms and salts thereof. The alkyl radi-
cals in the new compounds are preferably of low molecu-
lar weight; such groups are e.g. methyl, ethyl, straight-
chain or branched propyl, butyl, amyl or hexyl, cyclo-
pentyl or cyclohexyl. Methyl and isopropylradicals are
particularly advantageous. Apart from the alkyl radicals
at at least one ring nitrogen atom and the oxo (hydroxy)
group in at least one of the positions 4 and 6, these com-
pounds may be further substituted, for example, at one of
the nitrogen atoms and/or in 4- or =6-position. The 4- or .
6-position may for instance be occupied by a free or etheri-
fied hydroxyl or mercapto group, for example such a group
etherified with a lower alkanol, such as methanol, ethanol
or propanol, or a free or lower a1ky1ated,,e.g. methyl-
ated or ethylated amino group or halogen, such as chlo-
_rine, bromine or iodine. A ring nitrogen atom may carry
for example, an aminoalkyl radical, such as a_dilower al-
kyl-amino-lower alkyl e.g. dimethyl-, diethyl or dipropyl-
amino-ethyl or propyl radical, but more especially a lower
alkyl group, such as methyl, ethyl or isopropyl.
The new compounds have valuable pharmacological
properties, especially a caffein-like action, and can be used
as medicaments with stimulating and diuretic activity and
as intermediate products for the manufacture of medica-
ments having such activity. Especially valuable as diure-
tics are hydropyrazolo(3,4-d)pyrimidines havingthe nu-
cleus of the formula '
4
N5 3!
p,
N N/
which contain in at least one of the positions 4.and 6 an
oxo group and which are lower alkylated or oxyalkylated
or cycloalkylated at at least two ring nitrogen atoms, and _
also their tautomeric forms. .
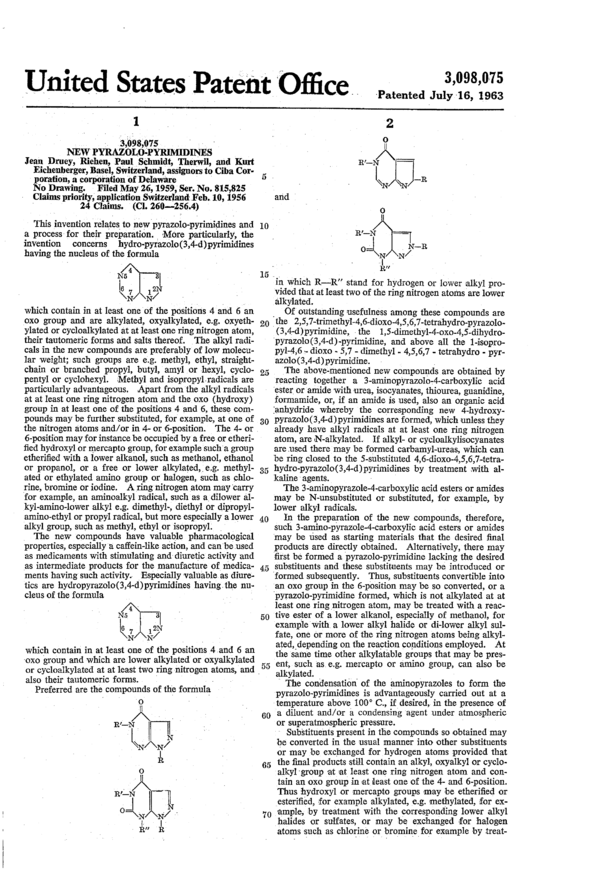
Preferred are the compounds of the formula
0
R’—N/l\ 4
- I
Q}
Ill.
0
I
R/—N
°=LN‘
ii.” it
10
15 I.
20:
25
30
35
40
45
50
60
65
70
2
‘u’
R,—_N/\
L\N/\N ‘R
and I . ‘
O
R'—N ——
04,
fill
in which R—R” stand for hydrogen or lower alkyl pro-
vided that at least two of the ring nitrogen atoms are lower
alkylated.
Of outstanding usefulness among these compounds are
the 2,5,7-trimethyl-4,6—dioxo-4,5,6,7-tetrahydro-pyrazolo-
(3,4.-d)pyrimidine, the 1,5—dimethyl-4-oxo-4,5-dihydro-
‘pyrazo1o(‘3,4-d)-pyrimidine, and above all the l-isopro-
pyl-4,-6 - dioxo - 5,7 - dimethyl - 4,5,6,7 - tetrahydro - pyr-
azo1o(3,4-d) pyrimidine.
The above-mentioned new compounds are obtained by
reacting together a 3—aminopyrazolo-4-carboxylic acid
‘ester or amide with -urea, isocyanates, thiourea, guanidine,
formamide, or, if an amide is used, also an organic acid
_‘anhydride whereby the corresponding new 4-hydroxy-
pyrazo1o(3,4—d)pyrimidines are formed, which unless they
already have alkyl radicals at at least one ring nitrogen
atom, are N -alkylated. If alkyl- or cycloalkylisocyanates
are used there may be formed carbamyl-ureas, which can
be ring closed to the 5-substituted 4,6-dioxo-4,5,6,7w-tetra-
hydro-pyrazolo(3,4-d)pyrimidines by treatment .with al-
kaline agents.
The 3-aminopyrazole-4-carboxylic acid esters or amides
may be N-unsubstituted or substituted, for example, by
lower alkyl radicals.
In the preparation of the new compounds, therefore,
such 3-amino-pyrazole-4-carboxylic acid esters or amides
may be used as starting materials that the desired final
products are directly obtained. Alternatively, there may
first be formed a pyrazolo-pyrimidine lacking the desired
substituents and these substituents may be introduced or
formed subsequently. Thus, substituents convertible into
an oxo group in the 6-position may be so converted, or a
'pyrazolo-pyrimidine formed, which is not alkylated at at
least one ring nitrogen atom, may be treated with a reac-
tive ester of a lower alkanol, especially of methanol, for
example with a lower alkyl halide or di-lower alkyl sul-
fate, one or more of the ring nitrogen atoms being alkyl-
atedhdepending on the reaction conditions employed. At
the same time other alkylatable groups that may be pres-
ent, such as.e.g. mercapto or amino group, can also be
alkylated.
The condensation" of the aminopyrazoles to form the
pyrazolo-pyrimidines is advantageously carried out at a
temperature above 100° C., if desired, in the presence of
a diluent and/or a condensing agent under atmospheric
or superatmospheric pressure.
Substituents present in the compounds so obtained may
be converted in the usual manner into other substituents
or may be exchanged for hydrogen atoms provided that
the final products still contain an alkyl, oxyalkyl or cyclo-
alkyl - group at -at least one ring nitrogen atom and con-
tain an oxo group in at least one of the 4- and 6-position.
Thus hydroxyl or mercapto groups -may be etherified or
esterified, for example alkylated, e.g. methylated, for ex-
ample, by treatment with the corresponding lower alkyl
halides or sulfates, or may be exchanged for halogen
atoms such as chlorine or bromine for example by treat-
3,093,075
3
ment with halides or phosphoric acid. Hydroxyl groups
may be exchanged for sulfur atoms for example by treat-
ment with phosphorus pentasulfide. Free or etherified
mercapto groups can be exchanged for amino or hydroxyl
groups for example by reaction with ammonia, primary or
secondary amines or hydrolyzing agents, respectively,
halogen atoms can be exchanged for hydroxyl groups or
etherified hydroxyl or mercapto groups or for amino or
hydrazino groups or hydrogen by reaction with hydrolyz-
ing agents, alcohols, mercapt-ans, amines, hydrazines or
appropriate hydrogenating agents, respectively. It is also
possible to introduce additional substituents. Non-alk-
ylated ring nitrogen atoms, for example, can be substituted
in any -desired manner, above -all by aminoalkyl radicals,
such as the dimethylaminoethyl radical, or alkyl radicals.
These subsequent reactions may be carried out in any
order and combination.
A preferred embodiment of the above subsequent re-
action consists in exchanging in a 6-hydroxy-5-a1kyl-4-
oxo-4,5-dihydro-pyrazolo(3,4-d)pyrimidine the hydroxyl
group for a halogen atom in the customary manner. This
is performed, for example, by treatment with a phosphoric
acid halide, such as phosphorus oxychloride or pentachlo-
ride or pentabromide. The thus obtained 5-alkyl-6-halo-
gen-4-oxo - 4,5 - dihydro-pyr‘azole(3,4 - d)pyrimidines are
new. They may bear substituents of any kind in the
pyrazole ring. In particular, they are substituted at one
of the two nitrogen atoms of the pyrazole ring by a lower
alkyl group, e.g. methyl or isopropyl. The alkyl radical
in the 5-position is more especially of a lower character,
such as for example a methyl or ethyl group, and the
halogen atom in 6-position preferably chlorine or bromine.
The new compounds have valuable properties. They
have an antibacterial and antimycotic activity. More par-
ticularly they have a coronary dilatating elfect. They can
therefore be used as medicaments or disinfectants. 8
Especially valuable 6-halogen compounds are 2,5-di-
methyl-4-oxo-6-chloro-4,5-dihydro-pyrazolo(3,4-d)pyrimi-
dine, 2-isopropyl-4-oxo-5 - methyl-6-chloro-4,5 - dihydro-
pyrazolo(3,4-d)pyrirr1idine, 1-isopropyl-4-oxo - 5 - ethyl-6-
chloro-4,5-dihydro - pyrazolo(3,4—d)pyrimidine and 1-iso-
propyl-4-oxo-5 - methyl-6 - chloro-4,5 - dihydro-pyrazolo-
(3,4-d)pyrimidine.
Owing to the reactivity of the halogen atom they are
also important intermediates for the manufacture of me-
dicaments. Thus they can be convertedinto 6-alkoxy com-
pounds by exchanging the halogen atom in the customary
manner. This is preferably carried out by treatment with
an alkanol, advantageously in the presence of a strongly
basic condensing agent, more especially one which is ca-
pable of forming salts with the alkanol, or with already
formed alkanolates, such as alkali alkanolates or alkaline
earth alkanolates. As condensing agents there come into
consideration more especially alkali metals or alkaline
earth meta-ls, their amides, hydrides, alcoholates or hydro-
carbon compounds.
The reaction is carried out in the usual manner in the
presence or absence of a -diluent, preferably at a raised
temperature.
The 5-alkyl-6 - alkoxy - 4 - oxo - 4,5 — dihydro-pyrazolo
(3,4-d)pyrimidines so obtained are new. They can be
substituted in any way in the pyrazole ring. In particu-
lar, they carry a lower alkyl group, for example methyl
or isopropyl, at one of the two nitrogen atoms of the
pyrazole ring. The alkyl radical in the 5-position is more
especially of a lower character, such as a methyl or ethyl
group. The radical in the 6-position is particularly a
lower alkoxy group, such as a methoxy group.
The new compounds have valuable pharmacological
properties. They exhibit a coronary dilatating and di-
uretic activity and can be used as medicaments.
Especially valuable are 1,5-dimethyl-4-oxo-6-methoxy-
4,5-dihydro-pyrazolo(3,4-d)pyrimidine, 1-isopropy1-4-oxo-
5-methyl.-6-methoxy-4,5 - dihydro-pyrazolo(3,4—d)pyrimi-
dine, 1-1sopropyl-4-oxo-5-ethyl-6-methoxy - 4,5 - dihydro.
U!
10
15
20
25
30
40
45
50
55
60
70
75
4
pyrazolo(3,4-d)pyrimidine, 2-isopropyl-4-oxo-5-methyl-6-
methoxy-pyrazolo(3,4-d)pyrimidine and 2,5-dirnethyl-4-
oxo-6—methoxy-4,5-dihydro-pyrazolo ( 3,4—d ) pyrimidine.
Depending on the substituents present in the final prod-
ucts they can be converted into salts. I-f they contain
free hydroxyl, mercapto or carboxyl groups, metal salts,
such as alkali metal, alkaline earth metal, or ammonium
salts, can be made, for example, by dissolving the prod-
ucts in appropriate alkaline solutions. Compounds of
basic character, such as those having basic substituents,
form therapeutically useful acid addition salts with ap-
propriate inorganic or organic acids. As salt-forming
acids there may be used, for example, hydrohalic acid,
sulfuric acid-, phosphoric acids, nitric acid or perchloric
acid; aliphatic, alicyclic, aromatic or heterocyclic carbox-
ylic or sulfonic acids, such as formic acid, acetic acid,
propionic acid, oxalic acid, succinic acid, glycollic acid,
lactic acid, malic acid, tartaric ‘acid, citric acid, ascorbic
acid, oxymaleic acid, dihydroxymaleic acid, pyroracemic
acid; phenyl-acetic acid, benzoic acid, para-aminobenzoic
acid, anthranilic acid, para-hydroxybenzoic acid, salicylic
acid or para-aminosalicylic -acid; methane sulfonic acid,
ethane sulfonic acid; toluene sulfonic acids, naphthalene
sulfonic acids or sulfanilic acid; and methionine, trypto-
phane, lysine or arginine. In the process of the invention
there are advantageously used starting materials which
lead to the formation of the pyrazolo-pyrimidines stated
above to be especially valuable.
The 3—aminopyrazoles used as starting materials and
containing in the 4-position an esterified carboxyl group
or the amide group can be obtained, for example, by re-
acting a substituted or unsubstituted a-cyano-;3-oxo-pro-
pionic acid ester or nitrile or an enol-ether, acetal or
mercaptal thereof, with a hydrazine. The latter is un-
substituted or mono-substituted, e.g. by an alkyl or cy-
cloalkyl radical. As functional derivatives of oc-cyano-B-
oxo-propionic acid there are advantageously used enol
ethers of oz-cyano-13-ox-o-propionic acid esters or nitriles,
for example, ethoxy-methylene-cyanoacetic acid ethyl
ester. The condensation to form the pyrazoles proceeds
mi-ld conditions, in part at room temperature and exo-
tthermically. It is -also possible to work at a higher tem-
perature and in the presence of a condensing agent, for
example, an .acid, -advantageously the reactants are re-
acted together in the presence of a diluent, such as an
alcohol, -toluene or chloroform. A nitrile group in the
resulting 3-amino-pyrazoles in which the substituent of
the hydrazine, if any, is in the 2-position may be hy-
drolyzed to the amide group in the usual manner. The
preparation of the starting materials is furthermore dis-
closed in our copending application Serial No. 637,897,
now Patent No. 2,965,643, and No. 637,898, now Pat-
ent No. 2,868,803, both filed February 4, 1957. 1-alkyl-
3-amino-pyrazoles of the above kind are obtained in the
manner, however using a hydrazine which besides the
desired substituent contains at the other nitrogen a sub-
stituent capable of being split off by hydrolysis. This
substituent is then split off from the open chain inter-
mediate forrned whereupon usually under the condition
of the hydrolysis, ring closure occurs.
The pyrazolo-pyrimidines described above, and their
salts, or mixtures of these compounds can be used as dis-
infectants, stimulants, or diuretics e.g. in the form of
pharmaceutical preparations. These preparations con-
tain the aforesaid compounds in admixture with a phar-
maceutical organic or inorganic carrier sutiable for en-
teral, parenteral or topical administration. As carriers
there are used substances that do not react with the afore-
said compounds, for example, gelatine, lactose, starches,
magnesium stearate, talc, vegetable oil, benzyl alcohols,
gums, polyalkylene glycols, cholesterol or another car-
rier known for medicaments. The pharmaceutical prepa-
rations may be made up, for example, in the form of
tablets, dragees or in liquid form as solutions, suspensions
or emulsions. If desired, they may be sterilized and/ or
may contain auxiliary substances, such as preserving,
3,098,075
5
stabilizing, wetting or emulsifying agents. They may
contain other therapeutically valuable substances. The
preparations can be made by the usual methods. This
is a continuation-in-pant of copending application Ser.
No. 775,344, filed November 21, 1958, by Jean Durey
and Paul Schmidt, and now abandoned, which is a con-
tinuation-in-part of application Ser. No. 718,438, filed
March 3, 1958, by Jean Durey and Paul Schmidt, and
now abandoned, which itself is a continuation-in-pant of
application Ser. No. 637,896, filed February 4,, 1957, by
Jean Druey and Paul Schmidt, and now abandoned.
The following examples illustrate the invention; the
quantities, unless given in grams or cc., -are given in parts
by weight.
Example 1
50 grams of 3-amino-4-carbethoxy—pyrazole and 100
grams of urea are mixed together well and heated for 40
minutes in a bath at 200° C. The melt is then extracted
with 400 cc. of a hot 1 N-s-olufion of caustic soda, the
mixture is filtered to remove a small amount of undis-
solved material, and the filtrate is rendered acid with
glacial acetic acid, and the wh-ite precipitate so formed
is filtered off with suction. There is obtained 4,6-dihy-
droxy-pyrazolo(3,4-d) pyrimidine in the form of white
crystals, which do not melt even -at 300° C.
A solution of 15.2 grams of 4,6-dihydroxy-pyrazolo
(3,4-d)pyrimidin-e in 200 cc. of a 2 N-solution of caustic
soda is slowly mixed, while stirring, with 42 grams of di-
methyl sulfate. The whole is then stirred for 20 hours
at room temperature, and the solution is extracted with
a large quantity of chloroform. The residue obtained by
evaporating the chloroform solution is recrystallized from
boiling water. There is obtained 2,5,7-trimethyl-4,6-
dioxo-4,5,6,7—tetrahydropyrazolo(3,4-d)pyrimidine of the
formula
0
ll
HaC——N
0% N—CH3
N/\N/
la.
in the form of white crystals melting at 195—196° C.
The 3-amino-4—carbethoxy-pyrazole used as starting
material can be prepared as follows:
8.5 grams of ethoxy-methylene-cyanacetic acid ethyl
ester are introduced into 50 cc. of alcohol. The solution
is then mixed with 2.5 cc. of hydrazine and the whole is
boiled for 6 hours under reflux. The whole is evapo-
rated to dryness in vacuo and crystallized from a small
amount of water. 3-amino-4-carbethoxy-pyrazole is ob-
tained in the form of while crystals melting at 102-
103 ° C.
The 2,5,7 — trimethyl-4,6-dioxo-4,5,6,7-tetrahydro-pyra-
»zolo(3,4-d)pyrimidine can be used as medicament with
caifein-like stimulating activity, e.g. in the form of
dragees having the following composition:
. Milograms
2,5,7—trimethyl-4,6-dioxo-4,5,6,7-tetrahydro-pyrazolo
3,4-d)pyrimidine ____________________ __'__..__ 100
Lactose ___________________________________ __ 86
Acrosit Compositum _________________________ -_ 30
Wheat starch _______________________________ __ 82
Arrowroot ____________________________ ..i._-__ 35
Magnesium stearate _________________________ __ 2
Talc _____________________________________ _- 15
350
The dragees are prepared in the usual manner.
‘Example 2
A mixture of 25 grams of 3-amino-4-carbethoxy-pyin
azole and 50 grams of thiourea is heated for one hours in
.a bath at 200° C. The melt is then taken up on 400
cc. of 2 N-solution of caustic soda and acidified with
C1
10
15
20
25
30
35
40
45
50
55
60
65
740
75
.10 hours at room temperature.
6
glacial acetic acid, whereupon 4—hydr'oxy-6—mercapto-pyr-
az,olo(3,4~d)Pyrimi:d-ine precipitates as white crystals,
which do not melt even at 300° C. _
16.8 grams of 4-hydroxy-6-mercapto-pyrazolo(3,4-d)-
pyrimidine are dissolved in 200 cc. of a 2 N-solution of
caustic soda, and the solution is slowly mixed, while
stirring, with 42 grams of dimethyl sulfate. The whole
is stirred for 20 hours at room temperature and then
extracted with a large quantity of chloroform. The
chloroform solution is evaporated and the -residue consists
of a mixture of two compounds, of which one is sparingly
soluble in ethyl acetate and is recrystallized from a large
quantity of alcohol. 2,5—dirnethyl-6-methylmercapto-4-
oxo-4,5—dihydro-pyrazolo(3,4-d)pyrimidine of the formula
0
I
H3C—N
N—CH3
HaCS— kN/
is obtained as white crystals melting at 203—204° C.
Example 3
7.5 grams of 3-amino-4-carbethoxy-pyrazole are mixed
with 30 cc. of formamide, and the mixture is then heated
for 8 hours in a bath at 190—20~0=° C. The whole in then
allowed to cool, whereupon a grey crystalline precipitate
is -formed, and the latter is filtered ofi with suction. The
precipitate is dissolved in dilute caustic soda solution,
the solution is agitated with charcoal and adjusted to
a pH value of 3-4 . with 2 N-hydrochloric acid,
whereupon a white precipitate is formed, and the latter
is crystallized from a large quantity of boiling water. The
4-hydroxy-pyrazolo(3,4-d)pyrimidine is obtained in the
form of white crystals which do not melt even at 350° C.
14 grams of 4-hydroxy-pyrazolo(3,4-d)pyrimidine -are
introduced into 150 cc. of a 2 N-solution of caustic soda.
The solution is slowly mixed, while stirring, with 30 grams
of dimethyl sulfate, and the whole is further stirred -for
The solution is then
extracted several times with a large quantity of chloro-
form, -and the combined residues, obtained by evaporating
the chloroform solution, are crystallized from a large
amount of boiling alcohol. There are obtained two com-
pounds, none of which is sparingly soluble in alcohol
-and melts at 287-289“ C., and the other of which dis-
solves easily in alcohol -and melts at 181-182“ C. The
latter compound -is 1,5-dimethyl-4-oxo-4,5-dihydro-pyr-
‘:azole(3,4-d)pyrimidine of the formula .
0
ll
IE[aC—N
Ki N
\N N/
(EH3
and the first 2,5-dimethyl-4-‘oxo-4,5-dihydro-pyrazolo-
(3,4-d)pyrimidine of the formula
0
ll
CH3-—N/\T“-‘T?’
Q N—CHa
\N/
Example 4
15 .2 grams of the 4,6 - dihydroxy - py-razolo(3,4 - d)
pyrimidine obtainable as indicated in Example 1 -are dis-
solved in 110 cc. of 2 N-caustic soda solution. In the
course of 4 hours, 25 grams of dimethylsulfate are added
dropwise to the resulting solution and stirring is continued
at room temperature -for 6 hours. The alkaline solution
is then extracted with a large quantity of chloroform, the
trimethyl derivative formed as by-product passing into
the chloroform phase. The aqueous solution is given
ao9ao75
?
a pH of 4, whereupon a white crystalline precipitate is
formed which is distilled in caustic soda solution and
then precipitated with glacial acetic acid. The resulting
precipitate is crystallized seve-ral times from boiling water
to obtain 4,6-dioxo-5,7-dimethyl-4,5,6,7-tetral1ydro—pyr—
azolo(3,4-d)pyrimidine of the formula
l
x”\
H3C———N
O_ N
N/\N/
I H
OH:
in the form of white crystals of melting point 280° C.
Example 5
15 grams of 4,6-dihydroxy-pyrazolo(3,4-d)pyrimidine
and 50 grams of pulverized phosphorus pentasulfide are
introduced into 800 cc. of pyridine and the mixture is
heated for 6 hours in a bath have a temperature of 130°
C. The pyridine is expelled under reduced pressure, the
resinous residue mixture with 600 cc. of ice-water, the
whole allowed to stand at room temperature for half an
hour, and then heated on the water bath for 2 hours.
After cooling, the precipitate is filtered off with suction.
It is dissolved while hot in dilute caustic soda solution,
treated with animal «charcoal, precipitated «by means of 2
N-acetic acid, filtered with suction, and washed with water
and alcohol. In this manner there is obtained 4-mercapto-
6-hydroxy-pyrazolo(3,4-d) pyrimidine in the «form of crys-
tals which do not melt even at 300° C.
A solution of 12.6 grams of 4-mercapto-6-hydroxy-
pyrazolo(3,4—d) pyrimidine in 150 cc. of 2 N-caustic soda
solution is mixed in the course of 2 hours with 31.5
grams of dimethyl sulfate. Stirring of the mixture is
continued for 6 hours at room temperature and the
alkaline solution is then extracted from a large quantity
of chloroform. The residue is recrystallized from a small
quantity of alcohol to obtain 2,7-dimethyl-4-methyl-
rnercapto-6-oxo-6,7-dihydropyrazolo(3,4-d) pyrimidine of
the formula
OH;
in the form of yellowish crystals of melting point 216-
218“ C.
Example 6
A ‘solution of 10 ‘grams of the 2,7-dime-thyl-4-methyh
mercapto-6-oxo-6,7-dihydro-pyrazolo(3,4-d) pyrimidine in
80 cc. of concentrated hydrochloric acid is boiled for 3
hours. It is then allowed to cool and filtered with suc-
tion to separate the white precipitate. The latter is then
recrystallized from -dilute alcohol. There is obtained 2,7-
dimethyl-4,6-dioxo-4,5,6,7 - tetrahydro-pyrazolo(3,4-d) py-
rimidine of the formula
0
I
HN
04 I ‘-0...
N/\\N/
(IJH;
in the form of white crystals melting at 325—‘327° C.
Example 7
10 grams of the 2,7-dimethyl-4-methylmercapto-6-oxo-
6,7-dihydno-pyrazo1~o(3,4-d)pyrirnidine obtainable accord-
ing to Example 5 and 100 cc. of liquid ammonia are
heated at 100° C. for 6 hours in a sealed tube. The grey
Cl
10
20
30
40
60
75
8
crystals obtained after evaporation of the ammonia are
recrystallized from alcohol and 2,7—.dimethyl-4-amino-6-
oxo-6,7-dihydro-pyrazolo(3,4-d) pyrimidine of the formula
CH3
obtained in the form of white crystals which do not melt
even -at 320° C.
When this substance is treated with alcoholic hydro-
chloric acid there is obtained its -monochloro hydrate
which melts at 312° C. with decomposition. In similar
manner other salts, such as the sulfate, perchlorate, nitrate
or methane sulfonate can be obtained.
Example‘ 8
A solution of 8 grams of the 2,5-dimethyl-6-methyl-
mercapto-4-oxo-4,5 - dihydro-pyrazolo(3,4-d)pyrimidine
obtained according t-o Example 2 in 70 cc. of concentrated
hydrochloric acid is heated to the boil for '3 hours. After
cooling, the resulting white precipitate is separated by
filtering with suction. It is crystallized from dilute alcohol
and there is obtained 2,5-dimethyl-4,6-dioxo-4,5,6,7-tetra-
hydro-pyrazolo(3,4-d) pyrimidine of the formula
in the form of white crystals which do not melt even at
330° C.
Example 9
In a sealed tu-be, 8 grams of the 2,5-dimethyl-6-methyl-
mercapto-4-oxo-4,5 - dihydro-py'razolo'(3,4-d)pyrimidine
obtainable according to Example 2 and 80 cc. of liquid
ammonia -are heated to 20 hours at 100° C. The am-
monia is expelled and the residue crystallized from a
large quantity of alcohol. There is obtained the 2,5-di-
methyl - 6 - amino-4-oxo-4,5-dihydropyrazolo(3,4-d)py-
rimidine of the formula
0
ll
H3C-—N
_ N—CH3
H2N \N/LN/
in the form of white crystals which do not melt even
at 320° C.
When this substance is treated with alcoholic hydro-
chloric -acid there is obtained its monohydrochloride
which melts at 298° C. with decomposition.
Example 10
9 grams of the 4,6-dioxo-5,7-dimethyl-4,5,6,7«tetrahy-
dro-pyrazolo(3,4-d)pyrimidine described in Example 4
‘are introduced into a solution of 1.3 grams of sodium in
200 cc. of ‘alcohol, and the suspension stirred for 1 hour
at room temperature. 6 ‘grams of chlorethyldimethyl-
amine are then admixed and the whole ‘boiled for 10 hours
while stirring. The reaction mixture is then evaporated
in vacuo. The residue is mixed with 100 cc. of 1 N-
caustic soda solution and extracted with chloroform. By
recrystallization of the residue from petroleum ether there
is obtained the 2-(,8-di-methylaminoethyl)-4,6-dioxo-5,7-
dimethyl-4,5,6,7-tetrahydro-pyrazolo(3,4-d)pyrimidine in
the form of white crystals of melting point 126—127° C.
Example I]
10 grams of 2-isopropyl-3-amino-4-carbamyl-pyrazole
and 20 grams of urea are mixed thoroughly and heated
3,093,075
9
for 1 hour in a bath having a nternperature of 200° C.
The hot melt is then introduced into 15 0 cc. of 1 N-caustic
soda solution, treated with animal icharcoal, and filtered
with suction. The filtrate is given a pH of 3 with hydro-
chloric acid, whereupon white crystals precipitate. By re-
crystallization of the precipitate from water there is ob-
tained 1-isopnopyl-4,6-dihydroxy~pyrazolo( 3,4-d) pyrimi-
dine of the formula
on
I
N/\N/
(En
0&3 \OH3
in the form of white crystals of melting point 286-287“
C. (with decomposition).
The 2-iso-propyl-3-amino-4-carbamylpyrazole used as
starting material can be prepared as follows:
A solution of 48:8 parts of ethoxymethylene-maloni-
trile in 500 parts by volume of alcohol is mixed with
30 grams of isopropyl hydrazine. The mixture is then
heated to the boil for 10 hours, evaporated to dryness
under reduced pressure, and the residue crystallized from
a large quantity of isopropyl ether. 2—isop=ropyl-3-amino-
’4-cyano-pyrazole is obtained -in this manner in the form
of white crystals of melting point 94-95“ C. 10 grams
of the compound thus obtained are mixed -with 200 cc.
of 2 N-caustic soda solution and 100 cc. of alcohol, and
the solution boiled for :3 hours. The alcohol is evapo-
rated under reduced pressure, the reaction mass allowed
to cool, and the precipitate separated by filtering with
suction. The precipitate is recrystallized from alcohol.
There is obtained 2-isopropyl-3-amino-4-carbamyl-pyn
azole in the form of white crystals of melting point 215-
216° C.
Example 12
A solution of 10 ‘grams of the 1-isopropyl-4,6-dihy-
droxy-pyrazolo(3,4-d)pyrimidine described in Example 11
in 75 cc. of 2 N-caustic soda solution is mixed slowly,
.while being stirred, with 14 grams of dimethyl sulfate.
The reaction mass is allowed to stand overnight and ex- ‘
tracted with chloroform in the morning. The chloro-
form residue is recrystallized from alcohol to obtain 1-
isopropyl - 4,6 - dioxo - 5,7 - dimethyl - 4,5,6,7 - tetra-
hydro-pyrazolo(3,4-d)pyrimidine of the formula
0
I
H30-N
1. ll-
0..
31113 bH(0H3)z
in the form of white crystals melting at 141-142° C.
Example 13
19.7 grams of 2-isopropyl-4-carbethoxy-3-amino-pyraz-
ole are heated -with 50 cc. of formamide for 4 hours in
a bath of 2»00‘—21()° C. After cooling, the reaction mix-
ture is taken up in 2 N—caustic soda solution, treated with
‘animal charcoal, and precipitated by adjusting the pH
to 3 with 2 N-hydrochloric acid. l-isopropyl-4-hydroxy-
pyrazolo(3,4-d)pyrin1idi-ne of a formula
(|)H
in
\
Cfia CH3
10
15
20
25
30
35
40
50
60
65
70
#5
"C.
10
is obtained in the form of crystals of melting point 197-
198” C.
The 2-isopropyl-4-carbethoxy-3-amino-pyrazole used as
starting material can be prepared as follows:
8.2 grams of isopropyl hydrazine are introduced into
a solution of 16.9 grams of ethoxymethylenecyano-acetic
acid ethyl ester in 100 cc. of alcohol and boiled for 12
hours. The reaction mass is then evaporated to dryness
and the residue distilled in vacuo. 2-isopropyl-3-amino-
4-carebethoxy-pyrazole passes over at 164—166° C. un-
der a pressure of 10 mm. and solidifies in crystalline form
in the receiver. The colorless crystals obtained melt at
46—48° C.
Example 14
9 grams of 5,7-dimethyl-4,5,6,7-tetrahydro-4,6-dioxo-
pyrazolo(3,4-d)pyrimidine are introduced into a solution
of 1.2 grams of sodium in 200 cc. of anhydrous ethyl
alcohol. The reaction mixture is stirred for 1 hour at
room temperature. 7 grams of chlorethyldiethylamine
are then added and the whole heated to the boil for 10
hours while stirring. After cooling, the precipitated salt
is removed by filtering with suction and the filtrate evapo-
rated to dryness. The residue, is mixed with 20 cc. of
3 N-caustic soda solution and extracted -with a large
quantity of chloroform. -By distilling olf the chloroform
and recrystal-lizing the residue from isopropyl ether there
is obtained the 2-(13-diethylaminoethyl)-S,7-dimethyl-4,6-
dioxo—4,5,6,7 - tetrahydro-pyrazolo(3,4 - d) pyrimidine of
melting point v85—87° C.
Example 15
9 grams of 1-isopropyl—4-hydroxy-pyrazolo(3,4-d) py-
rimidine are dissolved in 40 cc. of 2 N—caustic soda solu-
tion and mixed slowly, while being agitated, with 8 grams
of dimethyl sulfate. A white product precipitates and
is separated by filtration with suction. By recrystalliza-
tion from water there is obtained 1-isopropyl-5—m'ethyl-
4-oxo - 4,5 - dihydroxypyrazolo(3,4 - d) pyrimidine in the
form of white crystals melting at 162—163° C.
Example 16
10 grams of 2-methyl-3-amino-4-carbamyl-pyrazole and
20 grams of urea are thoroughly mixed and heated for
3 hours in a bath of 200° C. The hot melt is then poured
into 150 cc. of 1 N-caustic soda solution, treated with
animal charcoal, and filtered with suction. The filtrate
is given a pH of 3 with hydrochloric acid whereupon
white crystals separate. By recrystallization of the pre-
cipitate from a large amount of Water, the 1-methyl-4,6-
dihydroxy-ptyrazolo(3,4-d)pyrimidine is obtained in the
form of white crystals which do not melt when heated
to 300° C.
7.5 grams of dimethyl sulfate are added dropwise to
a solution of 4.2 grams of 1-methyl-4,6-dihydroxy-pyra-
zolo(3,4-d) pyrimidine in 30 cc. of 3 N—caustic soda solu-
tion, and stirring continued for 10 hours. The pH is then
adjusted to 9 with 2 N-caustic soda solution, which oper-a-
tion is followed by extraction with chloroform. The chlo-
reform residue is recrystallized from much alcohol. 1,5,
7 - trimethyl-4,6-dioxo-4,5,6,7-tetrahydropyrazolo(3,4-d)-
pyrimidine is thus obtained in the form of white crystals
of melting point 230—231° C.
The 2-methyl-3-a-mino-4-carbamyl-pyrazole used as
starting material can be obtained as follows:
A solution of 40 gvams of ethoxymethylene-malonitrile
-in 400 cc. of ethanol is mixed with 27 grams of methyl
hydrazine. The mixture -is -boiled for 10 hours, allowed
to cool, and the precipitated product separated by filtra-
tion. 2—methyl-3-amino-4-cyano-pyrazole is thus obtained
in the form _of white crystals of melting point 2l9—220°
10 grams of this compound are mixed with 200 cc.
of 2 N-caustic soda solution and 100 cc. of ethanol, and
the solution boiled for 3 hours. The ethanol is evaporated
under reduced pressure, the reaction mass allowed to cool,
3,098,075
11
and the precipitate separated by filtration with suction.
The product is recrystallized from ethanol. There is thus
obtained 2-methyl-3-amino-4-carbamyl—pyrazole in the
form of white crystals of melting point 232-234“ C.
Example 17
A mixture of 17.5 parts of 3-amino-4—pyrazole-carbox-
amide (obtainable by -condensation of ethoxymethylene-
malonitrile with hydrazine and hydrolysis of the 3-amino-
4—cyano-pyrazole with concentrated sulfuric acid) and 35
parts of urea is heated at 160-180“ C. for 90 minutes and
then cooled. The solid fusion product is washed with
water. 13 parts of the unpurified 4,6—dihydroxy-pyra-
zolo(3,4-d)pyrirnidine thus obtained are dissolved -in 50
parts of 10% aqueous sodium hydroxide solution and 100
parts of water by heating. The solution is then brought
to about 40° C. and treated with 90 parts of 95% ethanol
and 28.6 parts of bromoethane «and the mixture is refluxed
for one day. Then, at 2 hour intervals, 5 portions each
of 17 parts of bromoethane and 22 parts of 10% sodium
hydroxide are added. The reaction mixture is taken al-
most to dryness and the resulting residue is exhaustively
extracted with chloroform. The chloroform extract is
dried over anhydrous sodium sulfate, stirred with activated
carbon, filtered and taken to dryness under vacuum. The
residue is taken up in a solution containing 7 parts of
benzene to 3 parts of petroleum ether and applied to a
chromatography column containing 500 parts of alumina.
Elution of the column with benzene yields first in impurity
and later the 2,5,7-triethyl-4,6-dioxo-4,5,~6,7-tetrahydro-
pyrazolo(3,4-d)pyrimidine. ecrystallized from dilute
ethanol the compound melts at about 118—120° C. The
chromatography column described above is further de-
veloped with benzene solutions containing increasing con-
centrations of ethyl acetate. Elution with a 50% solu-
tion of ethyl acetate in benzene yields 5,7-diethyl-4,6-di-
oxo - 4,5,6,7-tetrahydropyrazolo(3,4-d)pyrimidine which,
crystallized from water, melts at about 205-207“ C. The
amount of this fraction can be increased by decreasing
the amount of bromoethane in the alkylation procedure.
«Example 18
A solution of 2.3 grams of sodium in 40 cc. of abso-
lute ethanol is added to a solution of 15.05 grams of cyclo-
hexylhydrazine hydrochloride in 50 cc. of absolute etha-
nol. 16.9 grams of ethoxy-methylene-cyanoacetic acid
ethyl ester, dissolved in 20 cc. of ethanol are added to
the reaction solution, and the whole is heated for 10 hours
at the boil. After cooling the mixture, the precipitated
sodium chloride is filtered off with suction, and the fil-
trate is evaporated to dryness. The crystalline residue is
triturated with water and filtered with suction. There
is obtained 2-cyclohexyl-3-amino-4-carbethoxy—pyrazole
melting at 112-114" C. After being recrystallized from
petroleum ether the melting point of the product rises to
115-116° C.
20 grams of 2-cyclohexyl-3-amino-4-carbethoxypyrazole
are heated with 50 grams of formamide for 6 hours in a
bath having a temperature of 200—2l0° C. After cooling
the mixture, 1-cyclohexyl-4-hydroxy-pyrazolo(3,4-d)-py-
rimidine crystallizes out. It melts lat 245—246° C.
Example 19
16.9 grams of ethoxy-methylene-cyanoacetic acid ethyl
ester and 8.8 grams of secondary-butyl-hydrazine are
heated in 100 cc. of absolute ethanol for 10 hours at the
boil. The mixture is then evaporated in vacuo, and the
residue is distilled in vacuo. 2-(secondary-butyl)-3-ami-
no-4-carbethoxy-pyrazole boils under 0.09 mm. pressure
at 105-107“ C.
10.5 grams of 2-(secondary-butyl)-3-amino-4-carbeth-
oxy-pyrazole -are heated with 25 cc. of formamide for 6
hours at 200—210° C. The reaction solution is cooled to
0° C. and there is obtained crystalline 1-(secondary-
CI
10
20
30
40
45
50
55
60
65
70
75
12
butyl)-4-hydroxy-pyrazolo(3,4-d)pyrimidine of the for-
mula
OH
I
C
/
Ha
/I-‘-‘l
C C2H5
melting at 174—175° C.
Example 20
86 -grams of unethyl-isopropyl ketone are added to a
solution of 50 grams of hydrazine hydrate in 500 cc. of 2
N-hydrochloric acid with stirring and ice-cooling. Hydro-
genation is then carried out at room temperature and 19
atmosphere gauge pressure with 2 grams of platinum oxide
-as catalyst. Within an hour 22.4 liters of hydrogen are
taken up, which corresponds to one mol of H2. The
catalyst is filtered oif with suction, the reaction mixture is
evaporated to dryness under reduced pressure and 500
cc. of concentrated sodium hydroxide solution are added
to the -residue, 3-hydrazino-2-.methy1-butane separating in
the -form of oil. For the purpose of purification the oil
separated in the separating funnel is distilled. 3-hy-
drazino-2-methyl-butane passes over at 39—44° C. under
11 of pressure.
21 grams of 3-hydrazino-Znmethyl-butane are added to
a solution of 24.4 -grams of ethoxy-methylene-malonic
wacid-dinitrile in 250 cc. of ethanol. The reaction mix-
ture is heated under reflux for 12 hours, allowed to cool
and the precipitate filtered with suction. After recrystal-
lization from ethanol there is obtained 2-[3’—methyl-butyl-
(2) ]-3-amino-4-cyano-pyrazole in the form of white crys-
tals melting at 167—168° C.
200 cc. of 2 N-sodium hydroxide solution and 100 cc.
‘of alcohol are added to 18 grams of 2-[3’—methyl-butyl-
(2’)]-3-amino-4-cyano-pyrazole and the solution heated
at the boil for 3 hours. The alcohol is evaporated under
reduced pressure, -the reaction mixture allowed to cool and
the precipitate suction-filtered. The latter is -recrystallized
from alcohol and there is obtained 2-[3’—methyl-butyl-
(2’)]-3-amino-4-carbamyl-pyrazole in the form of white
crystals melting at 227-—228° C.
10 grams of 2-[3’-methyl-butyl-(2')]-3-amino-4-car-
bavmyl-pyrazole are heated with 30 grams of formamide
for 5 hours in a bath at 200—210° C. After cooling, 1-
[3' - methyl-butyl-(2') ]4-hydroxy-pyrazolo(3,4-d)pyrimi-
dine of the formula
(')H
N l.
k\N‘N/
I
CH3——C—CII(CI~I;)2
11
crystallizes; the product melts at 190—192° C.
Example 2]
344 grams of diethyl ketone are added to a solution of
200 grams of hydrazine hydrate in 528 cc. of 7.57 N-
hydrochloric acid with stirring and ice-cooling. After
adding 270 cc. of ethanol the whole is stirred for 30
minutes. Hydrogenation is carried out at room tempera-
ture and under 130 atmospheres gauge pressure with 2
grams of platinum oxide as catalyst. Within 15 minutes
the quantity of hydrogen calculated for 4 mols, 89.6 liters,
is taken up. The catalyst is suction-filtered, the filtrate is
adjusted to pH 4 with 2 N-hydrochloric acid and the solu-
tion is concentrated under reduced pressure until crystal-
lization sets -in. 500 cc. of concentrated sodium hydroxide
solution are added with ice-cooling. Solid sodium hy-
3,098,075
13
droxide is added until isopentylhydrazine separates as an
oil. The oil is decanted oif, dried over sodium hydroxide
and distilled. Pentyl-3-hydrazine passes over between
102 and 109°C.
84.5 grams of ethoxymethylene cyanacetic acid ethyl
ester and 51 grams of pentyl-3-hydrazine are heated to
the boil in 500 cc. of absolute alcohol for 10 hours. The
whole is evaporated under -reduced pressure and the .
residue distilled in vacuo. 2-pentyl-(3’)-3-amino-4-can
bethoxy—pyrazole boils at 175° C. under 11 mm. of pres-
su-re.
22.5 grams of 2-pentyl-(3')-3-amino-4~carbethoxy-pyra-
zole are heated in 50 cc. of formamide at 200—210° C.
for 10 hours. After cooling, the reaction product is ex-
tracted with methylene chloride. The methylene chloride
solution is washed twice with water and then evaporated.
The resulting residue is dissolved in 2 N-sodium hydroxide
solution. By acidifying with 2 N-hydrochloric acid there
is obtained l-pentyl-(3')-4-hydroxy-pyrazolo(3,4-d)py-
rimidine of the formula
(1)11
N
K.
N
1lq/
CH
/ \
0 2H5 0 2H5
melting at 140‘—l41° C. On recrystallization from a mix-
ture of ether and petroleum ether the melting point is
raised to 142—143° C.
Example 22
336 grams of cyclopentanone are added to a solution of
200 grams of -hydrazine hydrate in 572 cc. of 7 N-hydro-
chloric acid with stirring and ice-cooling. Hydrogenation
is carried out at room temperature under 100 atmospheres
gauge pressure with 2 grams of platinum oxide as cata-
lyst. Within an hour -the quantity of hydrogen calculated
for 4 mols. 89.6 liters, is taken up. The catalyst is filtered
oil with suction, the filtrate is adjusted to pH 4 with 2 N-
hydrochloric -acid and the solution evaporated in vacuo
until crystallization sets in. 500 »cc. of concentrated so-
dium hydroxide .sol-ution are then added with ice-cooling.
Solid sodium hydroxide are then added until cyclopentyl
hydrazine separates as an oil. The latter distils at 60-
65” C. under 11 mm. of pressure.
67.6 grams of ethoxymethylene cyanacetic acid ethyl
ester and 40 grams of cyclopentyl hydrazine are boiled
under reflux in 400 cc. of absolute alcohol for 10 hours.
The solution is evaporated in vacuo and the residue dis-
tilled in high vacuum. 2-cyclopentyl-3-amino-4-carbeth-
oxy-pyrazole boils at 152° C. under 0.15 mm. of pressure.
The melting point of the compound is 64—66° C.
22.3 grams of 2-cyclopentyl-3-amino-4-carbethoxy-pyra-
zole are heated in 50 cc. of formamide at 200‘-210° C. for
10 hours. After cooling, the precipitated crystals are suc-
tion-filtered, dissolved in 1 N-sodium hydroxide solution,
filtered and the filtrate acidified with 2 N-hydrochloric
acid to pH 4, whereby 1-cyclopenty1-4-hydroxy-pyrazolo
(3,4-d)pyrimidine melting at 225-226“ C. separates.
Example 23
392 grams of cyclohexanone are added to a solution
of 200 grams of hydrazine hydrate in 572 cc. of 7 N-hy-
. drochloric acid with stirring and ice-cooling. Hydrogena-
tion is then carried out at room temperature and under
100 .atmospheres gauge pressure with 2 grams of platinum
oxide as catalyst. Within 30 minutes the quantity of hy-
drogen calculated for 4 mols, 8-9.6 liters, is taken up.
1000 cc. of ethyl alcohol are added in order to dissolve
the precipitated crystals. The catalyst is then filtered oif
10
15
20
25
30
35
40
45
50
455
60
65
170
75
14
with suction, the filtrate is adjusted to pH 4 and evap-
orated in vacuo until crystallization sets in. After cool-
ing, the precipitated crystals are filtered and the filtrate
mixed with 500 cc. of concentrated sodium hydroxide solu-
tion with ice-cooling. Solid «sodium hydroxide is then
added until cyclohexylhydrazines separates as an oil. The
latter distils at 77—80° under 12 mm. of pressure. The
resulting distillate is reacted immediately with alcoholic
hydro-chloric acid into the hydrochloride; melting point
112—113‘’ C. '
150.5 grams of cyclohexyl-hydrazine hydrochloride are
dissolved in 500 cc. of ethyl alcohol and a solution of 23
grams of sodium in 400 cc. of ethyl alcohol is added with
stirring to a solution of 122 grams of ethoxymethylene
malonic acid dinitrile, the temperature rising to about 45°
C. The mixture is then heated -at the boil for 10 hours,
allowed to cool -and the precipitated sodium chloride fil-
tered off. The filtrate is evaporated to dryness in vacuo.
The residue is dissolved in 200 cc. of ethyl alcohol, filtered
and the solution poured into 1400 cc. "of water with stir-
ring, 2-cyclohexyl-3-amino-4-cyanopyrazole which melts
-at 124—126° C., precipitating in the form of crystals.
5 7 ‘grams of 2-cyclohexyl-3-amino—4—cyano-pyrazole are
boiled under reflux -for 21/2 hours in 230 cc. of absolute
alcohol and 230 cc. of 2 N-sodium hydroxide solution.
After cooling, the crystals are suction-filtered. There is
obtained 2-cyclo-hexyl-3-amino-pyr-azole-4-carboxylic acid
amide melting at 267-268° C.
30 grams ‘of 2-cyclohexyl-3-amino-pyrazole-4-carbox-
ylic acid amide are heated at 200° C. for 11/2 hours with
60 grams of urea. After cooling, 1 N-sodium hydroxide
is added to the reaction product, a small quantity of un-
dissolved matter is filtered off, and the filtrate acidified
with 5 N-hydrochloric acid, whereupon a precipitate sep-
arates. The latter is taken up in dimethyl-formamide,
filtered and allowed to crystalline out. There is obtained
1 - cyclohexyl-4:6-dihydroxy-pyrazolo(3,4-d)pyrimidine
melting at 330° C. with decomposition.
Example 24
75 grams of 2-secondary buty-l-3-amino-4-cyano-pyra-
zole are boiled under reflux for 21/2 hours in 750 cc. of
absolute -alcohol and 1500 of 2 N-sodium hydroxide solu-
tion. The solution is then concentrated to a volume of
about 1000 cc. in vacuo at a temperature of 50° C. and
then cooled to 0° C. The separated crystals are filtered
off. There is obtained 2-secondary butyl-3-a.-mino-pyra-
zole-4-carboxylic acid amide melting at 19‘8—l99° C.
60 grams of 2-secondary butyl-3-amino-pyrazole-4- car-
hoxylic -acid amide are heated at 200° C. for 11/2 hours
with 120 grams of urea. After cooling, 2.5 N-sodium
v hydrox-ide solution is added to the react-ion product, any
undissolved material is filtered off and the filtrate acidified
to a pH value of 3 with hydrochloric acid of 27%
strength, whereupon crystals separate. The latter are
dissolved in alcohol, the solution is filtered, the filtrate
evaporated and water added, whereupon crystallization
sets in. There is obtained 1-secondary butyl-4:6-dihy-
droxy-pyrazolo(3,4-d)pyrimidine melting at 225-227” C.
Example 25
A solution of 11.6 grams of N1-isopropyl-N2-acetyh
hydrazine and 17 grams of ethoxymethylene-cyanacetic
acid ester, in 250 cc. of ethanol is boiled for 12 hours
under reflux. The ethanol is then evaporated in vacuo,
150 cc. of 8 N-alcoholic hydrochloric acid are added to
the oily residue containing [3-(N2-acety1-N1-isopropyl-hy-
drazino)-on-cyano»acry1.ic acid ethyl ester, and the whole is
boiled under reflux for 2 hours. The mixture is again
evaporated in vacuo, the residue is taken up in 2 N-
aqueous hydro—chloric acid, the solution is filtered to re-
’ move undissolved material, and its pH value is adjusted to
8 to 9 with caustic soda solution. The mixture is then ex-
tracted with chloroform and the residue obtained by evap-
orating chloroform is recrystallized from cyclohexane.
3,098,075
1 5
There is obtained 1-isopropyl-3-amino-4-carbethoxy-pyra-
zole of the formula
CzH5O O C-1 CH3
/
H2N_\ N—CH
\N/ \CH3
in the form of white crystals melting at 72—73° C.
19.7 grams of 1-isopropyl-3-amino-4-carbethoxy-pyra-
zole and 30 grams of urea are thoroughly mixed together
and heated for 2 hours in a bath at 200° C. The hot melt
is then introduced into 150 cc. of 1 N-solution of caustic
soda, and the mixture is treated with animal charcoal and
filtered with suction. The filtrate is -given a pH value of
1-2 with hydrochloric acid, whereupon a white product
precipitates. The latter is crystallized vfrorn a large quan-
tity of water, and there is obtained 2-isopropyl-4:6-dihy-
droxy-pyrazolo(3,4-d)pyrimidine melting at 280—282‘’ C.
2.8 grams of dimethyl sulfate are slowly added while
stirring, to a solution of 1.9 grams of 2-isopropyl-4:6-di-
hydroxypyrazolo(3,4-d)pyrimidir1e in 15 cc. of a 2 N-
solution -of caustic soda. The whole is allo-wed to stand
overnight and the precipitate that is formed is filtered off
with suction. The latter is recrystallized from a large
quantity of petroleum ether, and in this manner there is
obtained 2-isopropyl-4,6-dioxo-5,7-dimethyl-4,5,6,7-tetra-
hydro—pyrazolo(3,4-.d)pyrimidine of the formula
0
ll
CH3—-N ~* /0113
0% \ N—CH
1,‘7/ N/ \OH3
CH3
in the -form of white crystals melting, at 182—l84° C.
Example 26
3 grams of 1-ethyl-3-amino-4-carbethoxy-pyrazole, dis-
solved in 25 cc. of benzene are heated with 5.17 cc. of
ethyl isocyanate and 0.5 cc. of triethylamine in a closed
tube for 10 hours at 100° C. By evaporating the reaction
solution, there is obtained crude crystalline N-[1-ethyl-4-
carbethoxypyrazolyl - ( 3)] - N’ - ethyl - N’ - ethyl - car-
bamyl-urea of the formula
C 2H5— O 0 (Pi
CzH5HN—O C-N— o C—HN—L\ /N—C2H5
N
$.11,
The crude product, which melts at 83—84° C. after re- '
crystallization from a mixture of ether and petroleum
ether may be used as such for carrying out the follow-
ing ring closure:
1 gram of the crude N-[1-ethyl-4-carbethoxy-pyrazolyl-
(3)]-N’-ethyl-N’—ethylcarbamyl-urea is boiled under re-
flux in 10 cc. of a 2 N-solution of caustic soda for 7
minutes under reflux. After being cooled the aqueous
solution is extracted with ether and adjusted to a pH
value of 5 with 2 N-acetic acid, whereby 2:5-diethyl-4:6-
dioxo - 4,5,6,7 — tetrahydro-pyrazolo-(3,4-d)pyrimidine of
the formula
0
ll
CgH5——N
0% N—C2H5
\N/\N/
I-I
precipitates ‘out, melting at 257—258° C.
Example 27
10.9 grams of ‘1-isopropyl-3-amino-4‘-carbethoxy-pyraz-
ole are heated in 85 cc. of benzene with 12.6 grams of
methyl isocyanate and :1.66 cc. of triethylarnine at 100°
C. for 10 hours in a closed tube. The reaction solution
Cl
10
15
30
‘40
14>
C1
C7!
CI
60
65
70
16
is evaporated to yield an oily residue, which consists sub-
stantially of N-[1-isopropyl-4-carbethoxy-pyrazolyl(3)]-
N’-methyl—N’-methylcarbamyl urea of the formula
C2H5O OC—i' CH3
/
/N413
N 0 113
CH3—-IIN—0 C—lTI—0 C-—I'IN—\
CH3
6.1 grams of this crude product are mixed with 60 cc.
of a 2 N-solution of caustic soda and the whole is boiled
under reflux for 12 minutes. The aqueous reaction solu-
tion is extracted with ether, and the aqueous solution is
acidified with 2 ~N-acetic acid to a pH value of 4.5, where-
upon 2-isopropyl-4,6-dioxo-5-methyl - 4,5,6,7 - tetrahydro-
pyrazolo(3,4-d)pyrin1idine of the formula
0
ll
/\
om—N *7‘ cm
/
0% /L )I—CI\I
11} N om
melting at 232—233° C. precipitates.
The 1-alkyvl-pyrazoles used in the above example and
in Example 26 can be obtained as follows:
8 grams of N1-isopropyl-N2-benzylidene-hydrazine and
8 grams of ethoxy-methylene-cyanacetic acid ethyl ester
are heated in 50 cc. of benzene for 10 hours at 80° C.
After the solvent has been removed in vaeuo, the residue
is recrystallized from ethanol. There is obtained ,8-(NT
rbenzylidene-N1-is opropyl-hydrazino ) -at-cyano-acrylic ‘acid
ethyl ester in the form of yellow prisms melting at 118-
l20° C.
4 grams of [3-(N2-benzylidene-N1—isopropyl-hydrazino)-
oz-cyano-acrylic acid ethyl ester are boiled for 2 hours with
10 N-alcoholic hydrochloric acid; the alcohol is then re-
moved by distillation in vacuo. The residue is taken up
in 200 cc. of 2 N-hydrochloric «acid and the solution ex-
tracted with ether. After separating the aqueous solution,
the latter is rendered alkaline by adding 2 N-sodium hy-
droxide solution. The separated base is extracted with
ether. After drying and evaporating the ether, 1-iso-
propyl-3—arnino-4-carbethoxy-pyrazole of the formula
CzH5O O C-
H2N—L
\N/
CH;
/
N—CII
\
CH3
remains which recrystallizes from cyclohexrane in the form
of white lamellae melting at 72—73° C.
In a similar manner 1-ethyl-3-amino-4-carbethoxy-
pyrazole boiling at 111° C. under 0.06 mm. of pressure
can be obtained.
Example 28
19.7 grams of 1-isopropyl-3-amino-4-carbethoxy-pyra-
zole are heated in 50 cc. of formamide for 5 hours in a
bath having a temperature of 200—210° C. After cooling,
the crystalline precipitate is suction-filtered and crystal-
lized from "boiling ethyl alcohol for the purpose of purifi-
cation. 2 - isopropyl-4-hydro~xy-pyrazolo(3,4 - d)pyrimi-
dine is obtained in the form of crystals melting at
229—230° C.
Example 29
4.5 grams of 2-isopropyl - 4 - hydroxy-pyrazolo(4,4-d)
pyrimidine are added to 20 cc. of 2 N-sodium hydroxide
solution. 4 grams of dimethyl sulfate are slowly added
to the solution with stirring and the whole is stirred for 2
hours at room temperature. The precipitate is suction-
filtered and crystallized from benzene. 2-isopropyl-4-oxo-
5-methyl-4,5—dihydro - pyrazolo(3,4 - -d)pyrimidine is ob-
tained in the form of «white crystals melting at 209-210° C.
Example 30
3 grams of 1-ethyl-3-amino-4-carbethoxy-pyrazole are
3,093,075
17
dissolved in 7 cc. of formamide and heated for 10 hours at
200—220° C. in an oil bath. The crystals which separate
on cooling are suction-filtered, washed with ether and re-
crystallized from -alcohol. 2-ethy1- 4 - hydroxy-pyrazolo
(3,4-d)pyrimidine is obtained melting at 235-237“ C.
Example 31
A mixture of 5 grams of 1-methyl-3-amino-4-carbeth
oxy—pyrazole and 4 grams of urea is heated for 10 hours
at 170° C. The reaction product is dissolved in 20 cc. of
warm 2 N-sodium hydroxide solution. On adding dilute
hydrochloric acid to the alkaline solution, 2-methyl-4,6-
dihydroxy -pyrazolo(3,4 - «d)«pyrimidine precipitates. It
melts at >360° C.
Example 32
5 grams of dimethyl sulfate are added in portions to a
solution of 5 -grams of 2-methyl-4,6-dihydroxy-pyrazolo
(3,4-d)pyrimidine in 20 cc. of 2 N-sodium hydroxide solu-
tion at room temperature. After 30 minutes the aqueous
solution is extracted with chloroform. The residue re-
maining «after drying and evaporating the chloroform ex-
tract is -recrystallized from benzene. 2,5,7-trimethyl-4,6-
dioxo-4,5 ,6,7-tetrahydropwyrazolo( 3,4 - d)pyrimidine melt-
ing at 195—'196° C. is obtained.
Example 33
5 grams of 1-methyl-3—amino-4-carbethoxy-pyrazole and
4 grams of thiourea are heated for 10 hours at 170° C.
The reaction product is dissolved in 20 cc. of 2 N-sodium
hydroxide solution. After filtering through active char-
coal and adding dilute hydrochl-oric acid, 2-methyl-4-hy-
droxy—6—mercapto - pyrazolo(3,4 — d) pyrimidine is precipi-
tated melting -at >360” C.
Example 34
In a sealed tube, 20 g; of 1-methyl-3-amino-4-carbeth-
oxy-pyrazole in 85 cc. of benzene are heated at 100° C.
for 10 hours with 10 g. of methylisocyanate. When the
reaction solution has cooled, the crystals which have sepa-
rated are filtered off with suction. There is obtained in
this manner the N—(1-methyl-4-carbethoxy—7~nyrazolyl)-
N’-methyl urea of the formula
C2H5—OOC—:=:'
CHa—NH——C-—NH N—°H3
('3
of melting point 119° C.
21 g. -of the afore-described substituted urea are heated
at 90° C. for 30 minutes in 20cc. of 5 N-caustic soda solu-
tion. When the reaction solution has cooled, the pH is
adjusted to 1 with 2 N-hydrochloric acid, after which the
2,5-dimethyl-4,6-dioxo-4,5,6,7 - tetrahydro - pyrazolo(3,4—
d)pyrimidine separates in the form of white crystals of
melting point 342-344° C.
The 1-methyl-3-amino-4 - carbethoxy - pyrazole used as
starting material is obtained as (follows:
A solution of 65 grams of N1—methyl—N2-benzylidene-
hydrazine and 85 grams of ethoxy-methylene-cyano-acetic
acid ethyl ester in 500 cc. of benzene is boiled under re-
flux for 10 hours. A precipitate is formed which is fil-
tered and recrystallized from ethanol. ;3-(N2-benzylidene-
N1-methyl-hydrazino)-at-cyano—acrylic acid ethyl ester in
the form of faintly yellow crystals melting at 155-15 6° C.
is obtained.
80 grams of B-(N2-benzylidene-N1-methyl-hydrazino)-
on-cyano-acrylic acid ethyl ester are boiled under reflux for
2 hours with 10 N-alcoholic hydrochloric acid. The sol-
vent is removed by ‘distillation in vacuo. The residue is
taken up in 200 cc. of 2 N-‘hydrochloric acid and the acid
solution extracted with ether. After separating the aque-
ous layer it is rendered alkaline by adding 2 N-sodium hy-
droxide solution. The precipitated -base is extracted sev-
eral times with ether. A-fter separating, drying and evap-
orating the ether extract, the residue is distilled at 130°
LU!
10
15
20
25
30
35
40
45
50
55
60
65
70
7_5 ‘
18
C. -under 0.01 mm. of pressure, 1—methyl-3-arnino-4-car-
lbethoxy-pyrazole of the formula
C2H50 O C I
N—CH
H2N—LN/ 3
melting at 92-93‘ C. is obtained.
Example 35
19.7 grams of 2-isopropyl-3-amino-4-carbethoxy-pyraz-
ole, dissolved in 150 cc. of benzene, are heated with
28.4 grams of ethyl isocyanate and 3 cc. of triethylamine
for 10 hours at 100° C. in -a closed tube. Upon evap-
orating the reaction solution there is obtained crude
crystalline N- [2-isopropyl - 4 - carbethoxy-pyrazolyl-( 3 ) ] -
N’-ethyl-N-ethylcarbamyl—urea of the formula
C2Hs0OC --H
02H5HN—O o—N—o oHN:m N
e. N”
2 5 bn
Céa \CH3
The crude product, which melts at 129—l30° C. after
recrystallization from ether, may be used directly for
ring closure in the following manner: 16 grams of crude
N- [2-isopropyl-4-carb ethoxy-pyrazolyl- (3 ) ] -N’ - ethyl-N’-
ethy-lcarbamyl-urea are mixed with 1360 cc. of a 2 N-solu-
tion of caustic soda, and the whole is boiled under reflux
for 8 minutes in an oil bath having a temperature of 150°
C. After -being cooled, the reaction solution is given a
pH value of 4.5 by the addition of 2 N-acetic acid, where-
upon 1-isopropyl - 4,6 - dioxo-5-ethyl-4,5,6,7-tetrahydro-
pyrazolo(3,4-d)pyrimidine precipitates. It melts at 202-
204” C. —
When the 2-isopropyl-3-amino-4-carbethoxy-pyrazole is
reacted in the above manner with only one molecular
proportion of ethyl cyanate, there -is obtained an inter-
mediate product from which, after distilling off the start-
ing material at 127° CI under 0.99 mm. pressure, N-[2-
isopropyl-4-carbethoxy-pyrazolyl-(3 ) ]-N’—ethyl-urea melt-
ing at 129—130° C. (after crystallization from a mixture
of ether and petroleum ether), can be isolated. It can
be subjected to ring closure in the manner described
above.
Example 36
13.8 grams of 2-isopropyl-3-amino-4-carbethoxy~pyraz-
ole are heated in 105 cc. of benzene with 16 grams of
methyl isocyanate and 2.1 cc. of triethylamine in a closed
tube for 10 hours at 100° C. By evaporating the reac-
tion solution, there is obtained crude crystalline N-[2-iso-
propyl-4-carbethoxy-pyrazolyl- ( 3 ) ]-N’-methyl-N’—methyl-
ca.-rbamyl-urea of the formula
C2H5OOC
CH3—HN——O C——lIT—O O—HN ' N
CH3 4)
/ .
CH3 CH3
The crude product,'which melts at 145—146° C. after
recrystallization from alcohol, can be used directly for
ring closure as follows:
5 grams of crude N-[2-isopropyl-4-carbethoxy-pyraz-
olyl-(3)]-N’-methyl-N’-methylcarbamyl-urea are boiled
under reflux for 7 minutes in 50 cc. of a 2 N-solution of
caustic soda. The reaction solution is extracted with
ether and adjusted to a pH value of 4.5 with 2 N-acetic
acid, whereupon 1-isopropyl-4,6-dioxo-5-methyl-4,5,6,7-
tetrahydro-pyrazolo(3,4-d) -pyrimidine precipitates. It
melts at 235-236” C.
Example 37
3.64 grams of 2-methyl-3-amino-4-carbethoxy-pyrazole,
dissolved in 32 cc. of benzene, are heated with 4.92 grams
/m \z
3,098,075
19
of methyl isocyanate and 0.7 cc. of triethylamine in a
closed tube for 10 hours at 100° C. By evaporating the
reaction solution there is obtained crude crystalline N-[2-
methyl - 4 - carbethoxy-pyrazolyl — (3)] - N’-methyl-N5
methylcarbamyl-urea of the formula
C2H50 O C -'——T|
OHa—HN——O C—N—O C—HN N
I N/
CH3 l
on.
The crude product, which melts at 148-149“ C. after
recrystallization from -alcohol, can be used directly for
the subsequent ring closure as follows:
1 gram of crude N-[2-methyl-4-carbethoxy-pyrazolyl-
(3)]-N’-methyl-N’-methylcarbamyl-urea are boiled under
reflux in 10 cc. of a 2 N-solution of caustic soda for 7
minutes. The aqueous reaction ‘solution is extracted
with ether and then adjusted to a pH value of 5 with 2
N-acetic acid, whereupon 1,5-dimethyl-4,6-dioxo-4,5,6,7-
tetrahydropyrazolo(3,4 -d)pyrimidine precipitates. It
melts at 297—298° C.
Example 38
5 grams of 1-methyl-3-amino-4-carbethoxy-pyrazole
are heated with 15 cc. of formamide for 10 hours at 190°
C. After cooling to room temperature the precipitate is
filtered and recrystallized from water. 2-methyl-4-hy-
droxy—pyrazolo(3,4-d)pyrimidine is obtained in the form
of white crystals melting at 193 ° C.
Example 39
A solution of 5 grams of ethyl-isocyanate and 10
grams of 1-methyl-3—amino-4-carbethoxy-pyrazole in 50
cc. of benzene is heated at 100° C. in an autoclave for
6 hours. After evaporating the solvent in vacuo, a solid
residue remains which is recrystallized from ether-cyclo-
hexane. N-ethy1- N’ - (1 - methyl-4-carbethoxy-3-pyraz-
olyl)-urea of the formula
C2H5—O 0 (PF
__ __ _ _ N-CH;
O2Hs EN 00 HN \N/
is -obtained in the form of prisms melting at 112° C.
15 grams of this urea are heated at 90° C. in 100 cc.
of 5 N-sodium hydroxide solution for 1 hour. The solu-
tion is filtered, and by adding 2 N-hydrochloric acid 2-
methyl - 5 - ethyl-4,6—dioxo - 4,5,6,7 - tetrahydro-pyrazolo-
(3,4-d) pyrimidine of the formula
i
C 2H5—I‘l/\ T‘
0 J N— C H3
—\E/ w/
precipitates. After recrystallization from water the com-
pound melts at 303 ° C.
Example 40
60 cc. of phosphorus oxychloride are added to 3 grams
of 1,5 - dimethyl - 4,6-dioxo-4,5,6,7-tetrahydropyrazolo
[3,4-djpyramidine and boiled under reflux. After about
3 hours the substance is in solution, and boiling under
reflux is continued for another 5 hours. The reaction
solution is evaporated at a water jet pump at a tempera-
ture of about 60° C. The residue is poured on to ice, the
pH is adjusted -to 10 with 2 N-sodium hydroxide solu-
tion, and extraction is carried out with chloroform. The
chloroform solution is evaporated and the residue is re-
crystallized fr-om ether. There is obtained 1,5-dimethy1-
10
15
20
30
4.0
45
50
55
60
65
70
75
20
4-oxo-6-chloro-4,5 — dihydro-pyrazolo[3,4-d] pyrimidine of
the formula
0
ll
CH3-N
/\
..lN
N
$113
melting at 177—178° C.
Example 41
60 cc. of phosphorus oxychloride are added to 4 grams
of 1-isorpropyl-4,6-diox-o-5 - methyl-4,5,6,7 - tetrahydro-
pyrazolo(3,4-d)pyrimidine and heated for 8 hours at the
boil. After about 1 hour the substance is in solution.
For -the purpose of working up, the reaction solution is
concentrated in vacuo at a maximum temperature of 50°
C. The residue is poured on to ice, the pH is adjusted
to 10 with 2 N-sodium hydroxide solution, and extrac-
tion is carried out with ample ether. From the evapo-
rated ether solution there is obtained 1-isopropyl-4-oxo-
5-methyl-6-chlor-o-4,5 - dihydro - pyrazolo(3,4-d) pyrimi-
dine of the formula
0
H
CH3-N
/\
I
OH
/\
CH3 CH3
melting at 100-101 ° C. After recrystallization from
petroleum ether the melting point is raised to 103—104° C.
Example 42
4 grams of 1-isopropyl-5-ethyl-4,6-dioxo-4,5,6,7-tetra-
hydro-pyrazolo(3,4-d-)pyrimidine are boiled under reflux
with 25 cc. of phosphorus oxychloride for 8 hours. For
the purpose of working up, the reaction solution is evapo-
rated in vacuo at a temperature of 60° C. at the most.
The residue is poured on to ice, the pH is adjusted to
10 with 2 N-sodium hydroxide solution, and extraction is
carried out with ether. From the evaporated ether solu-
tion there is obtained 1-isopropyl-4-oxo-5-ethyl-6-chloro-
4,5-dihydro-pyrazolo-(3,4-d)pyrimidine of the formula
0
C2H5—I\I/l\’.""]
o1—h\NiN/N
/it
CH3 CH3
melting at 64—650° C. After recrystallization from pe-
troleum ether, the melting point rises to 65—66° C.
Example 43
4 grams of 2-isopropyl-4,6-dioxo-5-methyl-4,5,6,7-tetra-
hydro-pyrazolo(3,4-d)pyrimidine are heated at the boil
with 50 cc. of phosphorus oxy-chloride. After two hours
the substance dissolves, and boiling is continued for an-
other 6 hours. The reaction solution is evaporated in
vacuo at a maximum temperature of 50° C. The solu-
tion is then adjusted to pH=l0 with cooling and ex-
tracted with ample ether. From the evaporated ether
solution there is obtained 2-isopropyl-4-oxo-5-methyl-6-
3,093,075
21
chloro - 4,5 - dihydro-pyrazolo(3,4-d)pyrimidine of the
formula
‘T
CH3—N ‘: CH3
mi lee
\N/W wt.
melting at 14S~150° C. After recrystallization from
other the substance melts at 150-151” C.
Example 44
4 grams of 2,5»dimethyl-4,6-wdioxo-4,5,6,7-tetrahydro-
.pyrazolo(3,4-d)pyrimidine are boiled under reflux with
130 cc. of phosphorus oxyohloride for 19 hours. After
cooling, the undissolved starting material is separated
off and the filtrate evaporated. The residue -is poured on
to ice, rendered alkaline with 2 N-sodium hydroxide solu-
tion and extracted with chloroform. From the evapo-
rated chloroform solution there is obtained by recrystal-
lization from acetone 2,5-dimethyl-4-oxo-6-chloro-4,5-
«dihydro-pyrazolo(3,4-d)pyri.m.idine melting at 199—200°
C.
Example 45
A methylate solution, prepared -from 2.67 grams of
sodium in 85 cc. of methanol, is added to 2.3 grams of
1,5 - dimethyl-4=oxo-6-chloro-4,5-dihydro-pyrazolo(3,4-
d)pyrirnidine, and the whole is boiled for 1 hour under
reflux. The mixture is then evaporated to 30 cc., water
is added and -the solution is extracted with chloroform.
The residue obtained from the evaporated chloroform
solution is recrystallized from a mixture of ether and pe-
troleum ether. There is thus obtained 1,5-dimethyl-4-
oxo — 6 — metlioxy-4,5—dil1ydro-pyrazolo(3,4-d)pyrimidine
of the formula
0
ll
CH3-I\T/\|—TII\I
CH3O \\N N/
$3.
melting at 160-161° C.
Example 46
To 2.26 grams of 1-isopropyl-4-oxo-5-methy1-6-ch1oro-
4,5-dihydro-pyrazolo(3,4-d)pyrimidine there is ‘added a
sodium methylate solution prepared from 75 cc. of meth-
anol and 2.3 grams of sodium, and the whole is boiled
under reflux for 1 hour. The reaction solution is concen-
trated -to 25 cc., diluted with water and extracted with
ample chloroform. The residue obtained from the evapo-
rated chloroform solution is recrystallized from a mixture
of ether and petroleum ether with the addition of a little
alcohol. There is obtained 1-isopropyl-4-oxo-5-methyl-6-
metlroxy-4,5-dihydro-pyrazolo(3,4 - d)-pyrimidine of the
formula
0
I
CH3—N
W.
N/
/5?
CH3
CHaO— \N
CH3
melting at 158—159° C.
Example 47
To 2.4 grams of 1-isopropy1-4-oxo-5-ethyl-6-chloro-4,5-
dihydro-pyrazolo(3,4-d)pyrimidine there is added a so-
dium methylate solution prepared from 75 cc. of methanol
and 2.3 grams of sodium, and the whole is boiled under
reflux for 1 hour. The reaction solution is concentrated
5
10
15
20
25
30
35
40
45
50
55
60
65
70
75
22
to about 25 cc., diluted with water and extracted with
chloroform. The residue obtained from the evaporated
'chl-orofo-rm solution is recrystallized from petroleum
ether. There is obtained 1-isopropyl-4-oxo-5-ethyl-6-
methoxy-4,5-dihydropyrazolo(3,4-d)pyrimidine of the
formula
0
II
C 2H5-N
\z=J
CH30— \N
0-2
/13:
/
CH3 CH3
melting at 107.5—108.5° C.
Example 48
To 2 grams of 2-isopropyl-4-oxo-5-methyl-6-chloro
4,5-dihydro-pyrazolo(3,4-d)pyrirnidine there is added a
sodium methylate solution prepared from 2.04 grams of
sodium in 65 cc. of methanol, and the whole is boiled
for 1 hour under reflux. The reaction solution is con-
centrated to about 25 cc., diluted with water and extracted
with much chloroform. The residue from the evapo-
rated chloroform solution is recrystallized from acetone-
-petroleum ether. There is obtained 2-isopropyl-4-oxo-5-
methyl-6-methoxy-pyrazolo(3,4-d)pyrimidine of the for-
mula
melting at 192-193“ C.
Example 49
0.8 gram of 2.5-dimethyl-4-oxo-6-chloro-4,5-dihydro-
pyrazolo(3,4-d)pyrimidine is boiled under reflux for 1
hour with a solution of 0.93 gram of sodium in 30 cc. of
methanol. The reaction solution is concentrated, diluted
with Water and extracted with chloroform. From the
evaporated chloroform solution there is obtained 2,5-
dimethyl - 4 — oxo - 6 - methoxy - 4,5 - dihydro - pyra-
zolo(3,4-d)pyrimidine which crystallizes from acetone;
M.P.=176—177° C.
Example 50
20 grams of diethyl sulfate are added to a solution of
8 grams of 2-methyl-4,6-dioxo-4,5,6,7-tetrahydro-pyra-
zolo(3,4—d)pyrimidine in 60 cc. of 2 N-sodium hydroxide
solution, and the whole is stirred while being heated at
95° C. for 4 hours. On cooling, a precipitate is formed
which is filtered off and recrystallized from ethanol.
There is obtained the 2-methyl-7-ethyl-4,6-dioxo—4,5,6,7-
tetrahydropyrazolo(3,4-d)pyrimidine in the form of crys-
tals of melting point 255° C.
Example 51
A mixture of 10 grams of 1-([3-hydroxy-ethyl)-3-amino-
4-oarbethoxy-pyrazole -and 20 grams of urea is heated
at 180° C. for 6 hours. The solid reaction product is
dissolved in 2 N-sodium hydroxide solution, filtered, and
the filtrate is given a pH of 1 by adding 2 N-sodium
hydroxide solution. On prolonged standing, the 2-(,B-
hydroxy —ethyl) - 4,6 - dihydroxy - pyrazolo( 3,4 — d)py-
rimidine separates out. For purification, it is recrystallized
from water. Its melting point is at 292° C.
Example 52
10 grams of 1-(/3-hydroxy-ethyl)-3-amino-4-carbeth-
oxy—pyrazole «are heated at 160° C. for 4 hours with
30 cc. of formamide. A crystalline precipitate forms
Which, for purification, is sublirned at 200° C. under a
5,098,075
23
pressure of 0.05 mm. In this manner there is obtained
2 — (2 - hydroxy - ethyl) - 4 - hydroxy - pyraz0lo(3,4-d)
pyrimidine in the form of white crystals melting at
269° C.
Example 53
A suspension of 10 grams of ethylhydrazine-oxalate in
1310 cc. of absolute alcohol is added to 11.25 grams of
ethoxy-methylene-cyanacetic ester, dissolved in 30 cc. of
absolute alcohol, -and the mixture boiled under reflux
while being stirred for 10 hours. After cooling, the re-
action solution is filtered and the filtrate evaporated. The
residue is mixed with 2 N-sodium hydroxide solution and
extracted several times with ether. The ethereal solution
is evaporated and the residue subjected to fractional distil-
lation under a high vacuum. There is obtained in this
manner the 2-ethyl-3-amino-4-carbethoxy-pyrazole which
under a pressure of 0.6 mm. boils at 120—121° C.
18.3 grams of 2-ethyl-3-amino-4-carbethoxy-pyrazole
and 50 cc. of formamide are heated under a current of
nitrogen for 10 hours in an oil bath having a temperature
of 200-—2l0° C. Part of the formamide is then evapo-
rated and the reaction mass allowed to cool. The crystals
that separate are filtered oif, dissolved in 2 N-sodium hy-
droxide solution, and filtered after the addition of active
carbon. The filtrate is given a pH of 3,5 with 5 N-hydro-
chloric acid, after which the 1-ethyl-4-hydroxy-pyrazolo
(3,4-d) pyrimidine separates in the form of white crystals
of melting point 236—237° C.
What is claimed is:
II. A member of the group consisting of 4,S,6,7-tetra-
hydro-pyrazolo(3,4-d)pyrimidines of the formulae:
B1
II
R2-N
/\
I I
R41!
and .
R4
wherein R is a member selected from the group consisting
of hydrogen, lower alkyl, hydroxy-lower alkyl, di—lower
alkylamino-lower alkyl and cyclo-lower alkyl -and R2 and
R4 are members selected from the group consisting of
lower alkyl, hydroxy: lower alkyl, di—lower alkylamino-.
lower alkyl and cyclo-lower alkyl and R1 and R2 «are
members of the group consisting of oxo, imino and thiono,
but at least one of these stands for oxo, and their thera-
peutically useful acid addition salts.
2. A member of the group consisting of dihydro-
pyrazolo(3,4-d) pyrimidines of the formulae:
R1
I
R-2~—N
R:—L l N
\N I?I/
R
fir
/\
R2—N
10
20
30
40
60
65
70
75
It i”
R: R
and
Rs
I
N ._
_ ——R
R“\N/ \N/
in
and their tautomeric forms, wherein R is a member
selected from the group consisting of lower alkyl, hydro-
gen, hydroxy-lower alkyl, di—lower alkylamino-lower alkyl
and cyclo-lower alkyl, and R2 is a member selected from
the group consisting of lower alkyl, hydroxy-lower alkyl,
di—lower -alkylamino—lower alkyl and cyclo-lower alkyl,
and R1 is .a member selected from the group consisting of
oxo, imino and thiono, and R3 is a member of the group
consisting of hydroxy, amino, mercapto, lower alkoxy,
lower alkylamino and lower alkylmercapto but at least
one of R1 and R3 is a member selected from the group
consisting of oxo and hydroxy, .and their therapeutically
useful acid addition salts.
3. A member of the group consisting of pyrazolo(3,4-
d) pyrimidines of the formulae
R1
I
N I
N
Br‘ \N N/
I
R
and
R1
I
N —”
R2_ N——R
‘N/\‘N/
and their tautomeric forms, wherein R is a member
selected from the group consisting of lower alkyl, lower
hydroxy-alkyl, lower dialkylamino-lower alkyl and cyclo-
lower alkyl and R1 and R2 are members selected from
the group consisting of hydroxy, amino, mercapto, lower
alkoxy, lower ralkylamino and lower alkylmercapto but
‘at least one of these radicals is hydroxy, and their thera-
peutically useful acid addition salts.
4. 1 - R-5-R2-4,6-dioxo-4,5,6,7—tetrahydro-pyraz0lo-(3,
4-d)pyrimidine, wherein R and R2 are lower alkyl having
together -at least 3 carbon atoms.
5. 1 - methyl - 5-R2-4,6-dioxo-4,5,6,7—tetrahydro-pyra-
zolo(3,4-d)pyrimidine, wherein R2 is lower alkyl.
6. 2 - methyl - 5-R2-4,6—dioxo-4,5,6,7-tetrahydro-pyra:
zolo(3,4—d) pyrimidine, wherein R2 is lower alkyl.
7. 1 - methyl - 5-R2—7-R4-4,6-dioxo-4,5,6,7-tetrahydro-
pyrazolo(3,4-d)pyrimidine, wherein R2 and R4 are lower
alkyl.
8. 2 - methyl - 5-R2-7-R1-4,6-dioxo-4,5,6,7-tetrahydro-
pyrazolo(3,4-d) pyrimidine, wherein R2 and R1 are lower
alkyl.
9. 1 - isopropyl - 5-R2-4,6-dioxo-4,5,6,7-tetrahydropyra-
zolo(3,4-d) pyrimidine, wherein R2 is lower alkyl.
10. 1 - R1 - 5 - R2 - 4,6 - dioxo - 4,5,6,7 - tetrahydro-
pyrazolo-(3,4-d)pyrimidine, wherein R1 and R2 are lower
‘alkyl, R1 and R2 together having at least 3 carbon atoms.
11. 4,6 - dihyvdroxy - pyrazolo(3,4 - d)pyrimidine N-
substituted in the pyrazole nucleus by lower alkyl.
12. 5 - lower alkyl - 6 - halogeno - 4 - oxo - 4,5 - dihy-
3,098,075
25
dro-pyrazolo(3,4~d) pyrimidine N-substituted in the pyr-
vazole nucleus by lower alkyl.
13. Snlower alky-1-6-lower alkoxy—4-oxo—4,5«dihydro-
pyrazo1o(3,4-d)pyrimidine N-substituted in the pyrazole
nucleus -by lower »alkyl.
14. 5 - R2 - 4,6 - rdioxo — 4,5,6,7 - tetrahydro - pyraz-
o1o(3,4—d)pyrimivdine, wherein R2 is lower alkyl, and
which is N-substituted in the pyrazole ring -by lower
alkyl.
15. 1,5,7—trimethyl-4,6-dioxo-4,5,6,7-tetrahydropyraz-
olo(3,4-d)pyrimidine.
16. 2,5,7-trimethyl-4,6~dioxo-4,5,6,7-tetrahywdropyraz-
olo(3,4~d)pyri.midine.
17. 1-isopropyl-5,7-dimethyl—4,6-dioXo-4,5,6,7—teti
Coments go here:
- Log in to post comments