Surgical Adhesives
Folder:
Year:
Abstract:
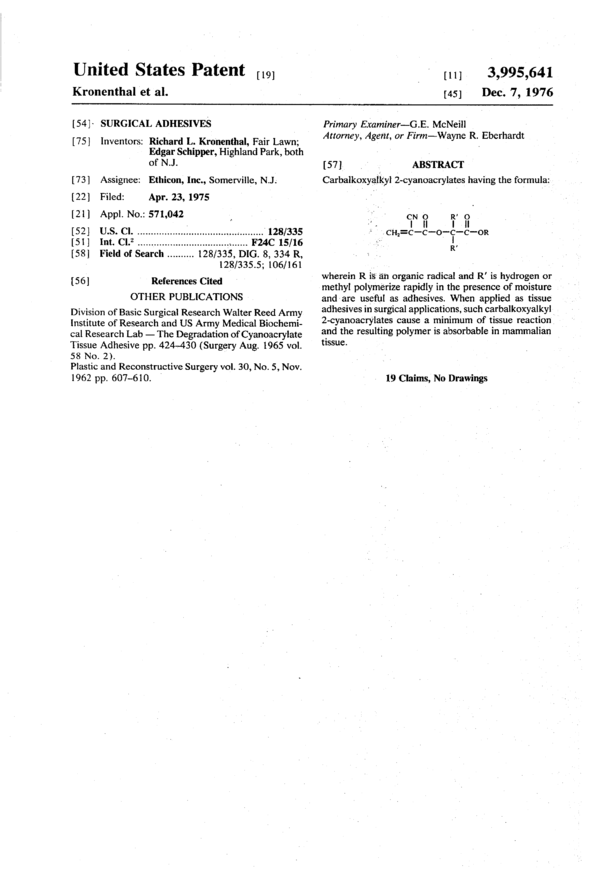
Carbalkoxyalkyl 2-cyanoacrylates having the formula: ##STR1## wherein R is an organic radical and R' is hydrogen or methyl polymerize rapidly in the presence of moisture and are useful as adhesives. When applied as tissue adhesives in surgical applications, such carbalkoxyalkyl 2-cyanoacrylates cause a minimum of tissue reaction and the resulting polymer is absorbable in mammalian tissue.
Type of document:
Language:
United States Patent [19]
Kronenthal et al.
I541‘
[75]
[73]
[22]
[21]
[52]
[51]
[58]
[561
SURGICAL ADHESIVES
Inventors: Richard L. Kronenthal, Fair Lawn;
Edgar Schipper, Highland Park, both
of N.J.
Assignee: Ethicon, Inc., Somerville, NJ.-
Filed: Apr. 23, 1975
Appl. No.: 571,042 I
U.S. Cl. ................. .§ .......................... .. 128/335
Int. CL? ................................ .... .. F24C 15/16
Field of Search ........ .. 128/335, DIG. 8, 334 R,
128/335.5; 106/161
References Cited
OTHER PUBLICATIONS
Division of Basic Surgical Research Walter Reed Army
Institute of Research and US Army Medical Biochemi-
cal Research Lab — The Degradation of Cyanoacrylate
Tissue Adhesive pp. 424-430 (Surgery Aug. 1965 vol.
58 No. 2).
Plastic and Reconstructive Surgery vol. 30, No. 5, Nov.
1962 pp. 607—6l0.
3,995,641
Dec. 7, 1976
[11]
[45]
Primary Examiner-—G.E. McNeill '
Attorney, Agent, or Firm—-Wayne R. Eberhardt
[57], ABSTRACT
Carbalkoxyalkyl 2—cyanoacrylates having the formula:
CN 0 12' o
I n I u
r .CH,=C—C—O'-(|2--C—0R
:
W
wherein R is an organic radical and R’ is hydrogen or
methyl polymerize rapidly in the presence of moisture
andare useful as adhesives. When applied as tissue
adhesives in surgical applications, such carbalkoxyalkyl
2-cyanoacrylates cause a minimum of tissue reaction
and the resulting polymer is absorbable in mammalian
tissue.
19 Claims, No Drawings
3,995,641
1
SURGICAL ADHESIVES
FIELD’ or THE INVENTION
This invention relates to a new class of cyanoacrylate
compositions which are useful as adhesives, and more
particularly to compounds which are carbalkoxyalkyl
2-cyanoacrylates. This invention further relates to the
use of carbalkoxyalkyl 2-cyanoacrylate monomers as
tissue adhesives in surgical applications.
DESCRIPTION OF THE PRIOR ART
Alkyl 2—cyanoacrylates, particularly the methyl, iso-
butyl and n—butyl 2-cyanoacrylates, have been investi-
gated for use asbiological adhesives, as reported for
example in Medical World News, 8 ,(20) 41 (1967);
Mfg. Chemist 38 (8), 9 (1967); Technical Report
6618, Walter Reed Army Medical Center, December,
1966. While the unsubstituted alkyl monomers appear
to possess the requisite bonding and hemostatic proper-
ties when applied to damaged tissues, these materials
appear to fail to have the required properties of low
toxicity and adequate absorption by the tissues. Methyl
2—cyanoacrylate, for example, gives“ rise to a severe
inflammatory tissue response at the site of application.
The n—butyl and isobutyl 2-cyanoacrylate monomers
are not absorbed well (if at all) by the tissues and poly-
meric residue of the adhesive has been observed by
histologic examination of the site of application as
much as twelve months after surgery as reported in
Medical World News, 8 (29), 27 (1967).
It is an object of the present invention to provide
cyanoacrylate monomers which are suitable for use in
biological adhesive compositions. More particularly, it
is an object of the present invention to provide cyano-
acrylate monomers which are polymerizable in the
presence of blood and other body fluids to form adhe-
sive bonds which do not significantly interfere with
natural healing of injured mammalian tissues, and
which are readily assimilated by the body with minimal
toxic effects.
A further object of this invention is to provide cyano-
acrylate monomers which can be used either alone or
as comonomers in the bonding of similar or dissimilar
materials without the use of heat or catalyst during the
bonding operation. Comonomer compositions are of
interest for specific uses because they may provide
advantageous combinations of properties not com-
pletely embodied in individual monomers.
A still further object of this invention is to provide
cyanoacrylate monomers which when cast in films, are
strong and flexible and particularly well suited for use
as wound and bum dressings.
Yet other objects of this invention will be apparent
from the description and claims which follow.
SUMMARY OF THE INVENTION.‘
The cyanoacrylates of the present invention are car-
balkoxyalkyl 2—cyanoacrylates of the general formula:
CNO R‘ o
I II I I
cH._,=c-C-0-cl-c—oR
. R,
5
l0
I5
20
25
30
35
40
45
50
55
60
65
2
wherein R is an organic radical having from 1 to about
12 carbon atoms and each R’ is individually hydrogen
or methyl. A particularly preferred class of compounds
is carbalkoxymethyl 2-cyanoacrylate.
Monomeric carbalkoxyalkyl 2—cyanoacrylates may
be employed individually or as comonomers in biologi-
cal adhesive compositions and exhibit excellent skin
wound adhesion and hemostatic properties. The poly-
meric products are readily assimilated by the tissues at
an acceptable rate and exhibit a relatively low degree
of inflammatory tissue response.
DESCRIPTION OF PREFERRED ‘EMBODIMENTS
The carbalkoxyalkyl 2—cyanoacrylates of the present
invention are those compounds described by the gen-
eral formula:
CNO R’ o
I II I II
CHz=C-C.—0—(lI—C-OR
R,
wherein R is an organic radical and each R’ is individu-
ally hydrogen or methyl.
Organic radical R is not critical to the instant inven-
tion and may be any hydrocarbon radical, substituted
or unsubstituted, which is convenient to the prepara-
tion and use of the carbalkoxyalkyl 2—cyanoacrylate
adhesives of the instant invention. Radical R may be
straight chain, branched or cyclic, saturated, unsatu-
rated or aromatic.
Typical examples of such organic radicals include
C,_3 alkyl radicals, CH alkenyl radicals, CH alkynyl
radicals, C3-” cycloaliphatic radicals, aryl radicals
such as phenyl and substituted phenyl and aralkyl radi-
cals such as benzyl, methylbenzyl and phenylethyl.
Also included are substituted hydrocarbon radicals,
particularly halo-, e.g., chloro-, fluoro— and bromo-sub-
stituted hydrocarbons, and oxy-, e.g., alkoxy substi-
tuted hydrocarbons. Preferred R radicals are alkyl,
alkenyl and alkynyl radicals having from I to about 8
carbon atoms, and halo substituted derivatives thereof.
Particularly preferred are alkyl radicals of 4 to 6 car-
bon atoms. '
Of the carbalkoxyalkyl 2—cyanoacrylates included
within the defined scope of the’ instant invention, car-
balkoxymethyl 2.-cyanoacrylates wherein each R’ is
hydrogen and R is an alkyl of from 4 to 6 carbon atoms
are particularly preferred for ease of preparation and
for efficacy as tissue adhesives. The ensuing specifica-
tion and examples are accordingly directed primarily
toward the preparation and use of such compounds, it
being understood that these examples are illustrative
only and not limiting of the instant invention.
PREPARATION OF CARBALKOXYMETHYL
2-CYANOACRYLATES
Carbalkoxymethyl 2—cyanoacrylates are prepared by
a reaction scheme in which the active vinyl group
I I
cH,=c—
of an alkyl 2—cyanoacrylate is first blocked by reaction
with a conjugated diene such as anthracene to fonn the
Diels - Alder adduct of the ester. The blocking group is
3,995,641
3
maintained during a subsequent two step transesterifi-
cation of the alkyl cyanoacrylate to a carbalkoxymethyl
cyanoacrylate, at which time removal of the blocking
group restores the active vinyl group. v
The general reaction scheme is illustrated in the,fol-
lowing flow chart in which® represents a cyclic 1,3-
diene blocking group.
Wherein R" is any alkyl or alkylene group, preferably
alkyl of l to 4 carbon atoms and R,R' and are as
defined above.
With specific reference to the above flow chart, an
alkyl esterof 2-cyanoacrylic acid (I) is reacted with a
cyclic 1,3-diene to form a Diels — Alder adduct (11).
Adduct (II) is subjected to alkaline hydrolysis followed
by acidification to form the corresponding 2-cyanoa-
crylic acid adduct (III). Adduct (III) is preferably ester-
ified by an alkyl bromoacetate to yield the correspond-
ing carbalkoxymethyl 2-cyanoacrylate adduct (IV).
Alternatively, adduct (111) may be converted to the
2-cyanoacrylyl halide adduct (III-A) by reaction with
60
65
4
thionyl chloride, and adduct (III-A) subsequently re-
acted with an alkyl hydroxyacetate or a methyl substi-
tuted alkyl hydroxyacetate to yield the corresponding
carbalkoxymethyl 2-cyanoacrylate adduct (IV) or car-
balkoxyalkyl 2-cyanoacrylate adduct (IV-A), respec-
tively. The blocking group is finally removed and ad-
duct (IV) or (IV-A) converted into the corresponding
FLOW CHART
|—-CH,
CIIN © /CN
® I I)KOH/ETOH/H20
cH.=c—CooR" ——9 c ——————9
‘ \ 2)HCl
COOR”
(I) (H)
'-—-CH., [—--CH_,
® /CN (1) /CN
BrCH.CO0R
L—c * L——c
\ (c._,H,.,),N \
coon CH2=C-C-O—Cl-COOR
pl—o—c|—cooR + R’ (v)
(IV-A) o R’ o
//
I--CH-C\
® /0
I-—-CH-C\\
(VI) 0
carbalkoxyalkyl 2-cyanoacrylate by heating the adduct
in the presence of a slight deficit of maleic anhydride.
A particularly preferred l,3—diene is anthracene, and
a typical anthracene adduct of Formula II is repre-
sented by the general structure:
H2
COC Ix“
\
COOR"
5
The preparation of alkyl esters of Zcyanoacrylic acid by
formation of intermediate Diels — Alder anthracene
adducts of the esters, and the subsequent removal of
the blocking anthracene group by heating the adduct
with maleic anhydride to yield the corresponding alkyl
2-cyanoacrylate monomer is described in U.S. Pat. No.
3,463,804, which patent is incorporated herein by ref-
erence. 1 '
The alkyl bromoacetates employed in the formation
of adduct (IV) ‘are prepared by the reaction of the
appropriate alcohols with bromoacetyl bromide in the
presence of dimethylaniline. Many of these compounds
have been described in .l. Agr. Food Chem., 6, 843
(1958). The esterification of an acid with alkyl brom-
oacetate is discussed in Helv. Chim. Acta, 38, 69
(1955). Both of these publications are incorporated
herein by reference. ‘ i
The preparation’ of the compounds of the present
invention is illustrated by the following examples which
are provided for purpose of illustration only and are not
limiting of theinvcntion. Unless otherwise noted, all
parts and percentages are by weight.
EXAMPLE 1
Preparation of .
9,10-Dihydro-9,10-ethanoanthracene-1 l-cyano-l 1-
carboxylic acid (Ill) .
A mixture consisting of 356.6 g (2.00 moles) anthra-
cene (98+%) and 306.4 g (2.00 moles) isobutyl 2-
cyanoacrylate in 2000ml of dry benzene previously
treated for 30 seconds with S0, was refluxed for 188
hours and cooled to room temperature. No unreacted
anthracene crystallized from the solution. The solution
was solvent stripped on a steam bath at water aspirator
pressures to yield a heavy slurry of crystalline solids.
Ethanol (500 ml) was added to the slurry and the sus-
pension stripped to a pasty solid residue. This process
was repeated with another 2 X 500 ml 95% ethanol in
order to strip off the bulk of the residual benzene. The
residue ‘was finally diluted with 2000 ml ethanol. A
solution of 195 g (3.00 moles) of potassium hydroxide
(86%) in 1000 ml H20 was then added. The reaction
mixture was stirred at amoderate reflux for two hours,
quenched in 7000 ml water, and the. precipitated an-
thracene (42.5 g, mp 212° —’2l6° C) filtered off after
standing at room temperature overnight. The filtrate
was acidified to pH 2.0 with 6N hydrochloric acid, and
the precipitated adduct was suction-filtered, washed
thoroughly with water, and air-dried to constant
weight. The yield ‘of anthracene 2-cyanoacrylic ‘acid
adduct (lll) was 482.3 g (88% theory), mp 200°—204°
C. 1
EXAMPLE 2
Preparation of lsobutyl bromoacetate
Over a period of 6-8 hours there wasadded dropwise
1 M of bromoacetyl bromide to a stirred solution of ‘l
M of isobutyl alcohol, 1 M of dimethylaniline and 200
ml of anhydrous ether. The solution was kept just
below boiling by adjusting the rate of addition and by
occasional cooling in an ice bath. After complete addi-
tion, the mixture was stirred overnight at room temper-
ature. Water (100 ml)-was added and the mixture
stirred until all of the precipitate had dissolved. The
layers were separated and the ether layer was washed
with 100 ml portions of 10% sulfuric acid until neutral-
ization of the washings resulted in a clear solution. The
5
'10
15
20
25
30
35
40
45
50
V55
60
65
3,995,641
6
washed ether solution was dried over anhydrous so-
dium sulfate and after removal of the drying agent and
solvent the residue was distilled in vacuo. The recov-
ered product had a bp / 14 mm = 72° — 76° C, n25 =
1.4468. - _ A
The following alkyl bromoacetates were prepared in
a like manner from their corresponding alcohols:
Octyl bromoacetate, bp / 0.5 mm = 86°—89° C, n?“ =
1.4560 - ' '
Hexyl bromoacetate, bp /0.5mm = 6l°—63° C, n25 =
1.4520 . » . . .
Benzyl bromoacetate, bp /0.09 mm = 78°—8l° C, n25
=_ 1.5425 _ L y . , .
~ n-Butyl bromoacetate, bp /0.8 mm = 37°—39° C, n.”“'5
= 1.4504 1 ,
2-Butoxyethyl bromoacetate, bp /1.2 mm = 83°—88°
c,n25=1.4849 1 ~ . .. :
2-Ethoxyethyl bromoacetate, bp /17' mm
109°-'1 10° C, r13‘‘-5 = 1,4575 ,
Allyl bromoacetate, bp /23 mm
1.4715
Cyclohexyl bromoacetate, bp / 1.6 mm = 75°—76° C,
n24 = 1.4847 , p 4 ‘
Propargyl bromoacetate, bp /33 mm ; 102°-103° C,
n23=1.4841 -. . . . 2 x =1.
Trifluoroethyl bromoacetate, bp./7 -mm = 34°—36° C,
n25-—-1.3935 “ " -*1 ‘
= 7§°—$o9 C, 1.26 =
_ EXAMPLE 3
General Procedure for. the Preparation of .
Carbalkoxymethyl «- 9, 10 - ,dihydro- 9,
l 0-ethanoanthracene- _l -carboxylates (IV)
To’a stirred solution containing molar quantities of
9, 10-dihydro-9, 10—ethanoanthracene- 1 1 -cyano-1 1‘-
carboxylic acid of Example 1 (hereinafter sometimes
referred to as “cyano acid”) and triethylamine in 2.25
l of dry ethyl. acetate, was added, dropwise over a period
of 30 minutes 1.1, M to 1.5 M of a selectedlalkyl brom-
oacetate dissolved in 500 ml of ethyl acetate. The mix-
ture was stirred and refluxed for 6 hours. Water (500
ml) was added in ‘order to dissolve the precipitate. The
layers ‘were separated, the aqueous layer was filtered
and the filtrate wasextracted with 500 ml of ethyl
acetate. The extract‘ was added to the original ethyl
acetate layer. The combined ethyl acetate layers were
washed with consecutiveeportions of the two -times
1500 ml of 1.2 M hydrochloric acid; saturated bicar-
bonate and water. The washed ethyl acetatesolution
was driegd-lover anhydrous sodium sulfate. The drying
agent was filtered off and the solvent removed in
vacuo. The residue, whenever possible,,was crystallized
by trituration with mixturespof ethyl acetate-hexane .
( 1:10) ‘or ether-pentane (1:10). (The octyl, 2'-ethox-
yethyl and 2’-butoxyethyl derivatives did not crystallize
and were used as their crude oils in the subsequent
deprotection step.).The crystalline products were re-
crystallized several times from ether or absolute alco-
hol; The products wereidentified by elemental analysis
and their IR. and NMR spectra. » '
The following specific compounds were prepared
according to the above procedure and using indicated
reactants.
3,995,641
7
A. Carbomethoxymethyl
9,1 O-dihydro-9, 1 0-ethanoanthracene- 1 1-cyano-1 1-
carboxylate
Reactants: 137.5 g (0.5 M) cyano acid, 114.8 g (0.75
M) methyl bromoacetate, 50.5 g (0.5 M) triethyl-
amine, 1375 ml ethyl acetate.
Mp l06°—108° C; Yield: 122.5 g (70.6%)
Analysis: Calc’d for C-_,,H,7O4N: C: 72.61, H: 4.93, N:
4.03. Found: C: 72.47, H: 5.03, N: 3.94.
B. Carbethoxymethyl
9,10-dihydro-9,10-ethanoanthracene-1 1-cyano-1 1-
carboxylate
Reactants: 137.5 g (0.5 M) cyano acid, 125.3 g (0.75
M) ethyl bromoacetate, 50.5 g (0.5 M) triethyl-
amine, 1400 ml ethyl acetate.
Mp 87°—88° C; Yield: 133.2 g (73.8%)
Analysis: Calc’d for C22H,9O.,N: C: 73.1 1, H: 5.30, N:
3.88. Found: C: 73.29, H: 5.35, N: 3.76.
C. Carbo-n—butoxymethyl
9,10-dihydro-9, l0—ethanoanthracene-1 1-cyano-1 1-
carboxylate
Reactants: 137.5 g (0.5 M) cyano acid, 121.9 g (0.625
M) n—butyl bromoacetate, 50.5 g (0.5 M) triethyl-
amine, 1.6 l ethyl acetate.
Mp 57°—58° C (recrystallized from ether-petroleum
ether); Yield: 92.0 g (47.3%)
Analysis: Calc’d for C2.,H-_.3O.,N: C: 74.02, H: 5.95, N:
3.60. Found: C: 74.29, H: 5.86, N: 3.56.
D. Carbo-i—butoxymethyl
9,10-dihydro-9,‘10-ethanoanthracene—1 1-cyano-1 1-
carboxylate
Reactants: 137.5 g (0.5 M) cyano acid, 146.3 g (0.75
M) isobutyl bromoacetate, 50.5 g (0.5 M) triethyl-
amine. .
The reaction residue from this reaction failed to crys-
tallize after the usual treatment and was taken up in
100 ml of ether. The solution was treated with charcoal
and filtered. The filtrate was diluted with 100 ml of
pentane. Crystals were obtained by allowing the solu-
tion to stand in an icebox and after scratching the in-
side of the reaction flask. After complete crystalliza-
tion, the mixture was filtered and the solids were re-
crystallized several times from absolute ethanol.
Mp 82°—83° C; Yield: 93.7 g (48.2%)
Analysis: Calc’d for C24H230.,N: C: 74.02, H: 5.95, N:
3.60. Found: C: 74.21, H: 5.96, N: 3.57.
E. Carbobenzoxymethyl 9,10-dihydro
9,10-ethanoanthracene- 1 1-cyano-1 1-carboxylate
Reactants: 55 g (0.2 M) cyano acid, 57.3 g (0.25 M)
benzyl bromoacetate, 20.2 g (0.2 M) triethylamine,
550 ml ethyl acetate.
Mp 75°-77° C (recrystallized from ether) Yield: 44.5 g
(52.7%).
Analysis: Calc’d for C27H2,O.,N: C: 76.58, H: 5.00, N:
3.31. Found: C: 76.74, H: 5.05, N: 3.30.
F. Carballoxymethyl
9,10-dihydro-9,10—ethanoanthracene-1 1-cyano-1 1-
carboxylate
Reactants: 171.3 g (0.623 M) cyano acid, 139.5 g
(0.779 M) allyl bromoacetate, 62.9 g (0.623 M)
triethylamine and :1 .6 1 ethyl acetate.
Mp 112°—113° C (recrystallized from ether) Yield:
167.3 g (71.9%).
5
10
I5
20
25
30
35
40
45
50
55
60
65
8
Analysis: Calc’d for C23 H,9O.,N: C: 73.98, H: 5.13, N:
3.75. Found: C: 74.09, H: 5.28, N: 3.80.
G. Carbopropargoxymethyl
9,10-dihydro-9,10-ethanoanthracene- 1 1-cyano-1 1-
carboxylate
Reactants: 164.5 g (0.6 M) cyano acid, 105.8 g (0.598
M) propargyl bromoacetate, 60.4 g (0.6 M) triethyl-
amine, and 1.6 1 ethyl acetate.
Mp 96°—97° C (recrystallized from ether) Yield: 131.5
g (59.6%). _
Analysis: Calc’d for C23H,-,-O.,N: C: 74.38, H: 4.61, N:
3.77. Found: C: 74.13, H: 4.54, N: 3.75.
H. Carbo-'2’,2’,2'-trifluoroethoxymethyl
9,10-dihydro-9,10-ethanoanthracene- 1 1-cyano-1 1-
carboxylate 1
Reactants: 110 g (0.4 M) cyano acid, 110.5 g (0.5 M)
2,2,2-trifluoroethyl bromoacetate, 40.4 g (0.4 M)
triethylamine and 1.3 1 ethyl acetate.
Mp 56°—5 7° C (recrystallized from ether—pentane 3:1)
Yield: 117.7 g (70.8%)
Analysis: Calc’d for C22H,,,O.,NF3: C: 63.61, H: 3,88, N:
3.37. Found: C: 63.92, H: 4.15, N: 3.25.
I. Carbohexoxymethyl
9,1 O-dihydro-9, 10-ethanoanthracene- 1 1-cyano-1 1-
carboxylate
Reactants: 206.3 g (0.75 M) cyano acid, 209.1 g
(0.938 M) hexyl bromoacetate, 75.8 g (0.75 M)
triethylamine, and 2.2 1. ethyl acetate.
Mp 55°—56° C (recrystallized from ether—pentane 1:1)
Yield: 201.2 g (64.3%).
Analysis: Calc’d for'C2sH27O4N: C: 74.80, H: 6.52, N:
3.36. Found: C:- 75.05, H: 6.69, N: 3.33.
EXAMPLE 4
General Procedure for the Preparation of
Carbalkoxymethyl 2-Cyanoacrylates (V)
A stirred mixture containing 0.25 M of a Carbalkox-
ymethyl 9,10-dihydro-9,10—ethanoanthracene- 1 1-
cyano-11-carboxylate of Example 3, 0.24 M of maleic
anhydride, 0.5 g hydroquinone and 1.0 g phosphorus
pentoxide in 250 ml of anhydrous xylene treated for 30
seconds with sulfur dioxide gas was refluxed for 5 — 18
hours. The anthracene—maleic anhydride was filtered
off and the filtrate was solvent stripped under reduced
pressure. Acetone (50 ml) was added, any solid matter
was filtered off, and the filtrate was again solvent
stripped. The residue was treated with 1.0 g of phos-
phorus pentoxide, subjected to vacuum sublimation at
0.1 mm Hg at a temperature not exceeding 100° C, and
finally vacuum distilled at 0.07 mm to 0.2 mm Hg abso-
lute pressure into a receiver containing about 0.05%
hydroquinone based on the projected yield of distillate.
The final product was stabilized with 300-600 ppm S02
and the hydroquinone concentration was adjusted to
0.1 percent. - . ,-
The following specific compounds were prepared
according to theiabove procedure and using the indi-
cated quantities of reactants. All products were identi-
fied via elemental analysis and IR and NMR spectra.
A. Carbomethoxymethyl 2-cyanoacrylate
Reactants: 122.5 g (0.353 M) Carbomethoxymethyl
9,10-dihydro-9,10-ethanoanthracene-1 1-cyano-1 1-
carboxylate, 94.1 g (0.339 M) maleicanhydride, 0.7
9
g hydroquinone. 1.4 g phosphorus pentoxide and 400
ml S03 treated xylene. Reflux time was 6 hours.
Bp (0.5 mm = 8-1°—86° C; Yield: 14.0 g (24%).
Analysis: Calcd for C-H;O4N: C: 49.71.. H: 4.17. N:
8.28. Found: C: 49.94. H: 4.32. N: 8.27. 5
B. carbethoxymethyl 2—cyanoacrylate
Reactants: 90.0 g (0.25 M) carbethoxymethyl-9.10-
dihydro-9.10-ethanoanthracene-1 1-cyano-1 l-ear-
boxylate. 23.5 g (0.24 M) maleic anhydride. 0.5 g 10
hydroquinone. 1.0 g phosphorus pentoxide. 250 ml
SO._. treated xylene. Reflux time was 8 hours.
Bp /0.5 mm = 89°—92° C: Yield: 22.7 g (50.5%).
Analysis: Calcd for C,.H._.O4N: C: 52.46. H: 4.95. N:
7.66. Found: C: 52.42. H: 4.84. N: 7.54.
C. Carbo-i-butoxymethyl 2-Cyanoacrylate (IX)
Reactants:.93.7 g (.241 M) carbo-i-butoxymethyl 9.10- '
dihydro-9.10-ethanoanthracene-1 1 -cyano— 1 1-car-
boxylate. 22.7 g (.232 M) maleic anhydride. 0.5 g
hydroquinone. 1 g phosphorus pentoxide and 250 ml
S02 treated xylene. The reflux time was 8 hours.
Bp /0.08 mm = 92°~96° C. On standing the distillate
crystallized. The crystals had a mp of 28°—29° C.
Yield: 32.5 g (66.6%) 3
Analysis: Calcd for C,.,H,;,O.N: C: 56.86. H: 6.20. N:
6.63. Found: C: 57.17. H: 6.00. N: (6.58.
D. Carbohexoymethyl 2-Cyanoacrylate
Reactants: 200.3 g (0.48 M) carbohexoxymethyl 9.10-
dihydro-9.10-ethanoanthracene-1 1-cyano-1 1-car-
boxylate. 45.2 g (0.461 M) maleic anhydride. 1 g
hydroquinone. 2 g phosphorus pentoxide and 400 ml
SO.1.treated xylene. The reflux time was 8 hours.
Bp /0.08 mm = 1l0°—113° C: Yield 50.9 g (44.9%).
Analysis: Calcd for C,2H1;O.,N: C: 60.24. H: 7.16. N:
5.85. Found: C: 6().l3. H: 7.27. N: 5.71.
IJ
Ln
30
35
E. C arboctoxymethyl 2-Cyanoacrylate
Esterificationof 137.5 g (0.5 M) of cyano acid with
157 g (0.625 M) of octyl bromoacetate. 50.5 g (0.5
M) of triethylamine and 1.3 1 ethyl acetate was car-
ried out according to the previously described gen-
eral procedure of Example 3. After workup, there
remained 186.9 g (0.42 M. 84.0%) of an oil which
did not crystalize. and which was subjected as such to
the reaction according to the general procedure of
Example 4 above.
Reactants: 39.6 g (.40 M) maleic anhydride. 0.8 g
hydroquinone. 1.6 g phosphorus pentoxide. and 400
ml 503 treated xylene. The reflux time was 8 hours.
The product was twice distilled.
Bp /0.07 mm = 119°—12l° C: Yield: 30.0 g (27.2%).
Analysis: Calcd for C,4H.;1O4N: C: 62.90. H: 7.92. N:
5.2-1. Found: C: 62.82. H: 7.79. N: 5.00.
40
45
50
55
F. Carballoxymethyl 2-Cyanoacrylate (X11)
Reactants: 140 g (0.375 M) carballoxymethyl 9.10- 60
dihydro-9.10-ethanoanthracene-1 1-cyano—1 1-car-
boxylate. 35.3 g (0.36 M) maleic anhydride. 0.75 g
hydroquinone. 1.5 g phosphorus pentoxide. and 400
ml of S02 treated xylene. The reflux time was 6
' hours.
Bp /0.()9 mm = 87°—90° C; Yield: 20.8 g (29.6%)
Analysis: Calcd for C9H9O4N: C: 55.38, H: 4.65. N:
7.18. Found: C: 55.70. H: 4.79. N: 7.06.
65
3,995,641
10-
G. Carbo—n-butoxymethyl 2-cyanoacrylate (X111)
Reactants: 83.2 g (0.214 M) carbo-n-butoxymethyl
9.10-dihydro-9.10-ethanoanthracene-1 1-cyano-1 1-
carboxylate. 20.1 g (0.205 M) maleic anhydride. 0.5
g hydroquinone. 0.9 g phosphorus pentoxide. and
230 ml. SO-_» treated xylene. Reflux time was 9 hours.
Bp /0.07 mm = 104°—105° C; Yield: 24.3 g.(53.8%).
Analysis: Ca1c'd for C1.,H1304N: C: 56.86. H: 6.20. N:
6.63. Found: C: 57.07. H: 5.90. N: 6.45.
H. Carbopropargoxymethyl 2-cyanoacrylate
Reactants: 131.5 g (.354 M) carbopropargoxymethyl
9. 10-dihydro-9.10-ethanoanthracene— 1 1-cyano-1 1-
carboxylate. 33.3 g (0.34 M) maleic anhydride. 0.7 g
‘hydroquinone. 1.4 g phosphorus pentoxide. and 350
ml S03 treated xylene. Reflux time was 7 hours.
Bp /0.07 mm = 94°—95° C: Yield: 7.5 g (11.4%).
Analysis: Calc'd for C9H7O4N: C: 55.96. H: 3.65. N:
7.25. Found:-C: 56.29. H: 3.71. N: 7.16.
1. Carbo-2',2',2'-trifluoroethoxymethyl
2-cyanoacrylate
Reactants: 100.1 g (0.241 M) carbo-2’,2',2’-trifluroe-
thoxymethyl 9.10-dihydro-9.10-ethanoanthrace.ne-
1l—cyano—11-carboxylate. 22.7 g (0.232 M) maleic
anhydride. 0.5 g hydroquinone. 1.0 g of phosphorus
pentoxide. and 200 ml S02 treated xylene. Reflux
time was 7 hours. The product spontaneously crystal-
lized during distillation. mp. 66°—67° C.
Bp /0.07 mm = 74°—75° C; Yield: 28 g (51.0%).
Analysis: Calcfd for C3H6O.,NF3: C: 40.52. H: 2.55. N:
5.91. Found: C: 4-0.6l.H: 2.51. N: 5.94. 1
.1. Carbo-2'-ethoxyethoxymethyl 2-cyanoacrylate
The crude anthracene adduct was obtained by the es-
terifieation of 162.4 g (0.59 M) cyano acid by 154.6
g (0.732 M) of 2'—ethoxyethyl bromoacetate in the
presence of 59.7 g (0.59 M) of triethylamine in 2 1
ethyl acetate according toithe general procedure of
Example 3. Yield of crude adduct: 222.5 g (0.549 M)
(93%). The crude adduct was reacted according to
the general procedure of Example 4 with the follow-
ing reactants:
Reactants: 51.6 g (0.527 M) maleic anhydride. 1 g
hydroquinone. 2 g phosphorus pentoxide. and 550 ml
S02 treated xylene. Reflux time was 8 hours. Yield.
after two distillations. 13 g_( 10.9%).
Analysis: Ca1c’d for C,oH,3O.-,N: C: 52.86. H: 5.77. N:
6.17. Found: C: 52.89. H: 6.00. N: 6.01.
K. Carbo-2'-n-butoxyethoxymethyl 2-cyanoacrylate
The crude anthracene adduct was obtained by the es-
terification of 192.0 g (0.698 M) Cyano acid. 194.5 g
(0.872 M) 2'-butoxyethyl bromoacetate in the pres-
ence of 70.5 g (0.698 M) of triethylamine and 2.4 1
ethyl acetate. according to the general procedure of
Example 3. Yield of crude adduct, 269.8 g (0.623 M)
(89%). This was reacted according to the general
procedure of Example 4 with the following reactants:
Reactants: 58.6 g (0.6 M) maleic anhydride. 1.3 g
hydroquinone. 2.6 g phosphorus pentoxide and 600
ml S02 treated xylene. Reflux time was 6 hours. The
reaction residue was distilled at 0.09-0.15 mm and a
portion boiling over a range of 117°—136° C was
collected. ‘This consisted of a mixture of solid and
liquid. The solid was filtered off and the filtrate was
subjected in part to two molecular distillations. The
3,995,641
11
material distilled at an outside temperature of
160°—1702 C and a pressure of 0.07 mm. IR and
NMR established the structure and GC gave a single
component purity of 96.6%.
EXAMPLE 5
Preparation of
9,10—Dihydro-9,l0—ethanoanthracene-1 I-cyano-1 1-
carbonyl chloride (III-A)
A mixture consisting of 27.5 g (0.1 M) of cyano acid
of Example 1, 14.5 ml (0.2 M) of thionyl chloride, 5
drops of pyridine and 200 ml of dry benzene is stirred
and refluxed under nitrogen for two hours. On cooling,
the cyano acid chloride adduct (III-A) is filtered off as
a crystalline product. washed with dry benzene, and
reacted with hydroxyacetate or a methyl substituted
hydroxyacetate to yield Compound (IV) or (IV-A)
respectively as indicated in the Flow Sheet. The reac-
tion thereafter follows the general procedure for reac-
10
15
tion of cyano acid with bromoacetate as set forth in 20
Example 3.
PRODUCT EVALUATION
Several of the cyanoacrylates of Example 4 were
evaluated for suitability as absorbable tissue adhesives
by determining hydrolytic stability of cyanoacrylate
polymers, bond strength using rat skin substrates, and
rate of absorption in rat subcutis. The heat of polymeri-
zation and the time required to reach maximum exo-
them was also determined for each adhesive in a mi-
crocalorimetry test since these parameters are signifi-
cant in certain tissue adhesive applications. Test proce-
dures and results are described below.
’ HYDROLYTIC STABILITY
Cyanoacrylate polymers were prepared by mixing
equal parts by weight of selected cyanoacrylate mono-
mers of Example 4 with reconstituted lyophilized
human plasma. The resulting polymer was washed,
dried, and pulverized to a powder. From 0.4 to 0.6
grams of powder was stirred into a buffered aqueous
solution prepared by dissolving 27 g monobasic sodium
phosphate in 1 liter of water and adjusted to a pH of
7.25 by the addition of 50% sodium hydroxide. The
temperature of the test solution was maintained at 37°
C and polymer weight loss was determined over a pe-
riod of three months with the following results:
Cyanoacrylate % Weight Loss
Monomer 1 wk 2 wks 1 mo 2 mos 3 mos.
a. MeC (Control) 1().7 14.1 19.3 28.8 44.0
b. IBC (Control) 3.9 2.4 2.4 2.8 5.2
c. CEC 13.5 18.6 27.5 38.3 51.3
d. CIBC 7.7 10.2 16.8 35.2 63.4
e. COC 2.3 4.1 19.4 55.9 85.6
MeC = methyl 2-cyanoacrylate
IBC = isnbutyl Z-cyanoacrylate
CEC = carbethoxymcthyl 2-cyanoacrylate
CIBC = curbo-i-butoxymcthyl 2-cyanoacrylate
COC = carboctoxymethyl 2-cyanoacrylate
As is apparent from the above data, the three cyano-
acrylates of the present invention (c, d and e) were
hydrolysed or converte_d to water soluble moieties at a
rate somewhat greater than MeC which is generally
considered to be quite absorbable, and at a rate consid-
erably higher than IBC, an adhesive of the prior art
which is considered to be substantially non-absorbable.
25
35
40
45
50
55
60
65
12
A BOND STRENGTH
Bond strength -in tissue adhesive applications was
determined with regard to the adhesion of freshly har-
vested rat skin. Strips of rat skin are mounted in holders
so that an area of approximately one square centimeter
of skin is exposed for adhesion. Two such holders are
mounted in opposing jaws of an Instron tensile testing
apparatus. One drop of adhesive is applied to the sur-
face of one rat skin, and the skins are’ brought into
contact and held under slight pressure for three min-
utes. The force required to separate the two skins at a
pull rate of 5 inches per minute is then measured and
the bond strength calculated in grams/cmz. The follow-
ing results were obtained.
Bond
» Strength
Adhesive glam’
2:. methyl 2-cyanoacrylate (MeC) 363
b. isobutyl 2-cyanoacrylate (IBC) 398
c. carbomethoxymethyl Z-cyanoacrylate (CMeC) 200
d. carbethoxymethyl 2—cyanoacrylate (CEC) 376
e. carbo-i-butoxymethyl 2-cyanoacrylate (CIBC) 330
f. carbo-n-butoxymethyl 2-cyanoacrylate (CBC) 405
g. carbohexoxymethyl 2-cyanoacrylate (CHC) 544
h. carboctoxymethyl 2—cyanoacrylate (COC) 328
i. carballoxymethyl 2-cyanoacrylate (CAC) 400
j. carbo-2'-ethoxyethoxymethyl
242
2-cyanoacrylate (CEOEC)
As is apparent’ from the above data, the bond
strength of most of the cyanoacrylates of the present
invention are comparable to those of MeC and IBC
prior art compounds. The CMeC compound tends to
be somewhat unstable and tests were performed with
partially polymerized material which accounts for the
lower bond strength. The CHC compound was unusual
in demonstrating exceptionally high bond strength.
MICROCALORIMETRY TESTS
This test measures the heat rise occurring when a 12
microliter drop of cyanoacrylate monomer is added to
a 100 microliter sample of plasma substrate contained
in thewell of a calorimeter. Heat rise is monitored to
determine maximum temperature increase and time to
reach that maximum. The following results were ob-
tained.
Heat of Polymerization
Adhesive ° C Time. Seconds
a. MeC — —
b. IBC 3.5 9
c. CMeC 2.0 128
d. CEC 3.0 20
e. CIBC 1.4 25
f. CBC 1.7 28
g. CHC 1.5 22
h. COC 1.1 17
i. CAC 1.7 29
j. 2.() 25
CEOEC
In the above test the MeC sample did not polymerize.
Of the remaining data, it is apparent that the adhesives
of the instant invention, particularly those of samples
(e) through (i), demonstrate significantly lower heats
of polymerization than the IBC control with somewhat
longer polymerization times.
_ _ .13
, A , iN'vivo’TEsTfsi, , _
The suitability of the cyanoacrylates of the instant
T invention for use_ as absorbable adhesives was finally
evaluated in limited in vivo tests in rats."Three to six
doses of 0.1 ml ‘each of selected monomers were in-
jected into the dorsal subcutisofa ‘group of forty rats.
The rats weresacrificed in groups" of five after times
ranging from 24 "hours to 6 months, and the "appearance
and weight of the polymerized cyanoacrylate was noted
together with a visual observation of tissue reaction.
The following data were obtained.
Percent Polymer Remaining
Tissue Weeks After Injection Appearance
Adhesive of ().l ml of Monomer At 4 Weeks
I 2 4 8 l2 I6 24
CMeC 50 () 0 — - — — Disintegruted
CEC — 52 lo — ——- —— —- Disintegrated Solid
CIBC —— 88 69 29 34 25 25 Disintegrated Solid
CNB 97 — 46 29 l2 l7 — Disintegrated Solid
CHC 92 — (ml 39 27 l7 5 Disinlegrated Solid
COC — 78 69 S() 40 26 12 Soft. Yellow mass
MeC —- 7] 52 38 30 I5 8 Disintegrated Solid
lBC -— 97 77 83 87 82 85 Unchanged
CEOEC — — 0 — — — — Disintegrated
It is apparent from the above data that the cyanoac-
rylates of the instant invention demonstrate good in
vivo absorbability, confirming the preliminary results
of the hydrolytic stability test.
The following observations regarding tissue reaction
to implanted cyanoacrylate adhesives were noted in
conjunction with the adhesive absorbability study:
TISSUE REACTION
Acute Hist-
otoxicit ‘ Encapsulation Thickness
Adhesive 6 Hr 24 Hr I Wk 4 Wks’ 2 M05
a. MeC 4 3 Medium Thick Thin
h. [BC I 0 ‘Thin Thin Thin .
c. CMeC 3 l Medium Medium — Thick Thin
d. CEC 3 l Medium Medium — Thick Thin
e. ClBC I 0 Thin Thin Thin
f. COC l 0 Thin Medium — Thin Thin
g. CHC l 0 Thin Thin Thin
In the above data, histotoxicity is rated visually from
no inflammation (rated 0) to significant inflammation
as generally associated with MeC (rated 4). It is thus
apparent from these data that the cyanoacrylates of the
instant invention, particularly the higher alkyl deriva-
tives, have a low order of histotoxicity comparable to
that commonly associated with non-absorbable IBC. It
is similarly apparent from the encapsulation thickness
data that the cyanoacrylates of the instant invention are
also generally comparable to [BC in degree of tissue
reactivity.
The preceding data illustrate that the cyanoacrylates
of the present invention are generally well suited as
absorbable tissue adhesives and demonstrate certain
functional advantages over the isobutyl 2-cyanoaery-
late of the prior art, particularly in regard to high ab-
sorbability associated with a low order of tissue reac-
tion.
The preceding examples and evaluations are pres-
ented for purposes of illustrating certain preferred em-
bodiments of the present invention. Many variations in
3,995,641.
5
I0
20
25
30
35
40
45
50
55
60
4‘ 14
these examples will be apparent to those skilled in the
art,“ and the invention is accordingly not limited to the
procedures,‘ reactants, or results desciibedtin’ the exam-
ples. _
I What is claimed is:
’ 1‘. A composition of the formula{
CH
coocH,co_oR
wherein® is a cyclic 1,3 diene and R is an organic
radical having from 1 to about 12 carbon atoms.
2. A composition of claim 1 wherein®is
3. A composition of claim 2 wherein R is alkyl, alke-
nyl, or alkynyl or a halo- or alkoxy-substituted alkyl,
alkenyl, or alkynyl group havingfrom l to about 8
carbon atoms, cycloalkyl, aralkyl, aryl or substituted
ar 1. '
ii. A composition of claim 2 wherein R is alkyl having
from 4 to 6 carbon atoms.
5. A composition of claim 2 wherein R is methyl,
ethyl, allyl, n-butyl, isobutyl, hexyl, oetyl, propargyl,
2-butoxyethyl, 2-ethoxyethyl, 2,2,2,-trifluoroethyl, cy-
clohexyl or benzyl. —
6. A composition of the formula:
°u”‘fi ‘i’
CH,=C-C-'0-ti‘-COOR
R’ 4
wherein R is an organic radical having from 1 to about
12 carbon atoms and each R’ is individually hydrogen
or methyl. .
7. A composition of claim 6 wherein R is alkyl, alke-
nyl, or alkynyl or a halo or alkoxy-substituted alkyl,
alkenyl, or alkynyl group having from I to about 8
carbon atoms, cycloalkyl, aralkyl, aryl or substituted
aryl. , — p
8. A composition of claim 6 wherein each R’ is hy-
drogen.
9. A composition of claim 8 wherein R is alkyl having
from 4 to 6 carbon atoms.
10. A composition of claim 8 wherein R is methyl,
ethyl, allyl, n-butyl, isobutyl, hexyl, octyl, propargyl,
2-butoxyethyl, 2-ethoxyethyl, 2,2,2,-trifluoroethyl, cy-
clohexyl or benzyl. ’
11. A method of joining together two surfaces which
comprises applying to at least one of said surfaces a .
compound of claim 6 and maintainingthe surfaces in
contact until said compound polymerizes.
65
12. A method of claim 11 wherein said surfaces com-
prise body tissue.
13. A method of claim 11 wherein one of said sur-
faces is body tissue and the other surface is a prosthetic
device.
3,995,641
A 15
14. A method for repairing damaged living tissue to
prevent the escape of fluids therethrough which com-
prises sealing said tissue with a film of a composition of
claim 6.
15. A method for stemming the flow of blood from
small vessels which comprises sealing said vessels with
a hemostatic agent comprising a composition of claim
6.
16. A method of dressing burns to promote the heal-
ing thereof which comprises covering said bum with a
precast film of a polymerized composition of claim 6.
5
l0
30
35
16
17. A method of dressing wounds to promote the
healing thereof which comprises covering said wound
with a precast film of a polymerized composition of
claim 6. ’
18. A method of dressing bums to promote the heal~
ing thereof which comprises coating the surface of said
burn with a composition of claim 6. '
19, A method of dressing wounds to promote the
healing thereof which comprises coating the surfaceof
said wound with a composition of claim 6.
=l< * * * *
406
45‘
L1-
u-
60
Page 1 of 2
UNITED STATES PATENT OFFICE
CERTIFICATE OF CORRECTION
Patent No. -2 N13‘ Dated December“ 7 1975.3
Inventor(s) i~‘~;iohaI‘d L. Kronenthal et al.
It is certified that error appears in the above—ident:'Lfied patent
and that said Letters Patent are hereby corrected as shown below:
In Column 6, Title of Example 3, "lO-ethanoanthracene-ll-
carboxylates IV)" should read -—— lO-ethanoanthracene-11-
cyano_carooxy ates (Iv) ---_
In Column 8, Line 23, "H: 3.88" should read --— H: 3.88 --—.
In Column 9, Line 29, "Carbohexoymethyl" should read --—
Carbohexoxymethyl —-—.
In Column 9, Line #7, "crystalize" should read ---
crystallize --—.
In Column 9, Line 55, "C: 62,90" should read --— C: 62.90 —-—.
Page 2 of 2
UNITED STATES PATENT OFFICE
CERTHHCATE OF CORRECTUUN
Patent No. 3z995,ES1+’! Dated Decembefz" ’,7 ’|‘;—?',f€_':
InVentor(s) i
Coments go here:
- Log in to post comments