Absorbable Tissue Adhesives
Absorbable Tissue Adhesives
US5350798
Folder:
Year:
Abstract:
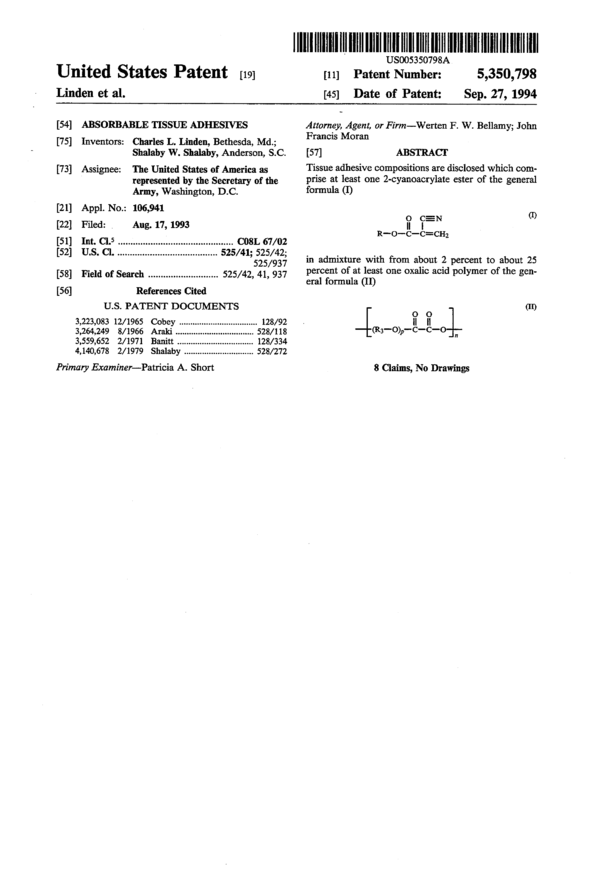
Tissue adhesive compostions are disclosed which comprise at least one 2-cyanoacrylate ester of the general formula (I) in admixture with from about 2 percent to about 25 percent of at least one oxalic acid polymer of the general formula (II).
Type of document:
Language:
United States Patent [19]
Linden et al.
USO05350798A
[11] Patent Number:
[45] Date of Patent:
5,350,798
Sep. 27, 1994
[54] ABSORBABLE TISSUE ADHESIVES
Charles L. Linden, Bethesda, Md.;
Shalaby W. Shalaby, Anderson, S.C.
The United States of America as
represented by the Secretary of the
Army, Washington, D.C.
[21] Appl. No.: 106,941
[7 5] Inventors:
[7 3] Assignee:
[22] Filed: , Aug. 17, 1993
[51] Int. Cl.5 ............................................ .. C08L 67/02
[52] U.S. Cl. ...................................... .. 525/41; 525/42;
525/937
[58] Field of Search .......................... .. 525/42, 41, 937
[56] References Cited
U.S. PATENT DOCUMENTS
3,223,083 12/1965 Cobey ................................. .. 128/92
3,264,249 8/1966 Araki 528/118
3,559,652 2/1971 Banitt ..... .. 128/334
4,140,678 2/1979 Shalaby ............................. .. 528/272
Primary Examiner—Patricia A. Short
Attorney, Agent, or Firm——Werten F. W. Bellamy; John
Francis Moran
[57] ABSTRACI‘
Tissue adhesive compositions are disclosed which com-
prise at least one 2-cyanoacrylate ester of the general
formula (I)
‘.1 15“ “’
R-O-C—C=CH2
in admixture with from about 2 percent to about 25
percent of at least one oxalic acid polymer of the gen-
eral formula (II)
(II)
0 0
II II
(R3—o),,—-c—c—o n
8 Claims, No Drawings
5,350,798
1
ABSORBABLE TISSUE ADHESIVES
FIELD OF THE INVENTION
This invention relates to improvements in the surgical
repair of mammalian body tissues. More particularly the
invention relates to improved surgical repair systems
comprising fast-polymerizing 2-cyanoacrylate mono-
mers modified by the addition of certain polymeric
oxalates.
BACKGROUND OF THE INVENTION
For many years surgical tissue closure has been ac-
complished by a variety of fundamental techniques such
as the use of clamps, staples or a variety of sutures.
Disadvantages associated with use of those techniques
has led to the development of new techniques for join-
ing damaged mammalian tissues and reducing or pre-
venting the loss of blood or other bodily fluids as well.
One approach has been the development of tissue
adhesives for joining tissues, derived from either natural
or synthetic products. Adhesive bonding with natural
products such as fibrin or glues derived from mollusks
such as mussels and barnacles has shown promise. Fi-
brin glue has been prepared by reacting a cryoprecipi-
tate of fibrinogen and thrombin in the presence of cal-
cium ion to produce fibrin monomer. This monomer
reacts in the presence of a factor found in the patient’s
blood (Factor XIII) to form a polymer. These fibrin
glues have found use in topical and spray applications as
a hemostatic agent on bleeding anastomoses, bleed
points caused by needle holes or suture lines, and on the
heart surface to control bleeding. The fibrin glues have
only a modest tensile strength and therefore have not
found significant use for repairing tissues which are
subjected to load.
Barnacle glue has shown promise since its polymeri-
zation is rapid and occurs under conditions which are
similar to the environment in which they would be
used. It also maintains its adhesive properties under
adverse chemical conditions. However under typical
use conditions the resulting adhesive joint has unaccept-
able tensile strength. Preparation of glues from mollusks
is difficult however, and large quantities of material
5
10
15
20
25
30
35
40
must be processed to obtain a significant amount of 45
adhesive. To prepare 1 milligram of adhesive from bar-
nacles requires the harvest and treatment of at least 150
barnacles.
For these reasons a great deal of attention has been
given to the development of synthetic adhesive systems.
Especially prominent has been the development of ad-
hesive and hemostasis-inducing compositions compris-
ing fast curing monomers such as dialkyl methylene
malonates (U .S. Pat. No. 3,221,745) and monomeric
lower alkyl 2-cyanoacrylates (US. Pat. Nos. 3,223,083
and 3,264,249). Because the lower alkyl 2-cyanoacry-
lates did not appear to combine the desired, if not neces-
sary, properties of low toxicity and adequate adsorption
by tissues, the use of alkoxyalkyl 2-cyanoacrylates was
developed (U.S. Pat. No. 3,559,652). Other polymers
presently under investigation include polyurethanes and
epoxy resins. The latter two polymer systems suffer
disadvantages of limited “pot life” or “open time”, have
significant exotherms when polymerized and exhibit
toxicity to surrounding tissues.
It is advantageous for tissue adhesives to able to be
absorbed or degraded in the body, otherwise known as
bioabsorption or biodegradation. Among the advan-
50
55
60
65
2
tages are that it has been shown that long-term implants
of nondegradable films and disks in rodents will induce
neoplasms, and although there are no studies to show
this will occur in primates, it is a matter for concern.
Second, it is obviously more desirable that a device used
in vivo should only remain as long as necessary to en-
sure proper healing. This should reduce or prevent
adverse tissue reactions and/or foreign body responses.
In orthopedic applications absorbable pins and plates
that could perform in place of metal implants would
require only a single surgical procedure. Absorbable
polymers would also be useful for use with implantable
systems for long-term drug delivery. The absorption
ability of current materials ranges from the least degrad-
able materials such as ceramics and carbon fibers
through metallic alloys to the most degradable, organic
polymers having reactive chains.
Shalaby in Encyclopedia of Pharmaceutical Technol-
ogy, Swarbrick and Boylan, eds., Marcel Dekker Inc.,
New York, 1988, pp. 465-476 has classified bioabsorba-
ble polymers into three groups; soluble, solublizable and
depolymerizable. Soluble polymers are water-soluble
and have hydrogen-bonding polar groups, the solubility
being determined by the type and frequency of the polar
group(s). Solublizable polymers are usually insoluble
salts such as calcium or magnesium salts of carboxylic
or sulfonic acid-functional materials which can dissolve
by cation exchange with monovalent metal salts. Depo-
lymerizable systems have chains that dissociate to sim-
ple organic compounds in vivo under the influence of
enzymes or chemical catalysis.
The response of tissues to biodegradable materials is
dependent on the rate of absorption, but more impor-
tantly it is regulated by the toxicity of the degradation
products. Thus it is important to have controlled ab-
sorption to decrease the toxicity and reaction of sur-
rounding tissue to products that do elicit a response. It
also is important to ensure that the mechanical proper-
ties of the polymer are maintained for sufficient time to
allow proper healing. Thus absorbable polymeric adhe-
sives and the products of their bioabsorption must be
compatible with the surrounding tissues.
2-cyanoacrylates bond rapidly and form strong adhe-
sive joints. Their properties may be modified easily by
modification of their substituent groups. They are well-
suited for biological applications since, unlike other
adhesives such as epoxy resins and polyurethanes, 2-
cyanoacrylates may be used as pure monofunctional
monomers having well-defined properties. They
homopolymerize rapidly at room temperature in the
presence of weakly basic moieties such as water and
other weakly basic species present in bodily fluids.
Since their introduction in 1958 they have found use in
many surgical applications such as hemostasis, as seal-
ants, for retrofilling and as general tissue adhesives. A
2-cyanoacrylate suitable for use as a tissue adhesive
should be non-toxic and biodegradable, should wet and
spread on tissue substrates and polymerize quickly to a
thin. polymeric film. The polymeric adhesive should
have a degree of flexibility, especially when bonding
soft tissues. Biodegradability is especially important
because the adhesive should be replaced by the body’s
tissues and not slow or bar complete healing.
In the homologous series of poly(alkyl 2-cyanoacry-
lates) the lower homologs such as the methyl ester ex-
hibit the highest rate of bioabsorption but also elicit the
greatest tissue response. They also do not wet, spread or
5,350,798
3
polymerize on biological substrates as rapidly as the
higher homologs. On the other hand, the higher alkyl
esters such as the isobutyl ester elicit minimal tissue
reaction but degrade slowly if at all. Therefore the main
drawbacks for use of the alkyl 2-cyanoacrylates has 5
been their histotoxicity and/or lack of biodegradability.
Despite those deficiencies, the n-butyl and isobutyl and
other higher esters have been found acceptable as tissue
adhesives. In an effort to combine the higher biodegrad-
ability of the lower alkyl esters with the lower toxicity
of the higher esters Banitt and Nelson (U.S. Pat. No.
3,559,652) developed alkoxyalkyl 2-cyanoacrylate ad-
hesives which were stated to be bioabsorbable and to
exhibit minimal toxicity and inflammation. Kronenthal
and Schipper (U.S. Pat. No. 3,995,641) developed a
carboxyalkyl 2-cyanoacrylate which was stated to be
useful as an adhesive or a wound dressing.
Other problems which have been observed with alkyl
2-cyanoacrylate adhesives are their low monomer vis-
cosities and the formation of a high modulus crust on
soft tissues. Due to their low molecular weight and
rapid polymerization times 2-cyanoacrylates may be
formulated with biologically acceptable modifiers. Be-
cause the monomer initiates with any anionic or free
radical source, formulations with modifiers are not eas-
ily made. Control of the viscosity of the monomeric
adhesive may be obtained by adding a biologically ac-
ceptable thickening agent. Millet has reported that
polylactic acid is an effective thickening agent (Struc-
tural Adhesives: Chemistry and Technology, S.R. Harts-
horn ed., Plenum Press, New York, 1986, pp.249—303)
Plasticizers are commonly used to decrease the brit-
tleness of polymers. Plasticizers function by lowering
the glass transition temperature and the modulus of the
polymer. Plasticizers may be internal or external. Inter- 35
nal plasticization is accomplished by using mixtures of
compatible monomers to form a copolymer having
segments of varying hardness. External plasticization
may be obtained by the addition of esters such as cyan-
oacetates, malonates, adipates, sebacates and the like 40
(Millet, op. cit.).
Therefore it is clear that there is a need for tissue
adhesives which have been modified by plasticizers/-
modifiers which exhibit biodegradability, an acceptable
histotoxicity and reasonably match the modulus of the 45
tissues being joined by the adhesive.
SUMMARY OF THE INVENTION
2-cyanoacrylate-based tissue adhesives have been
developed which employ biocompatible oxalate poly-
mers as reactive plasticizers and thickening agents. The
adhesives are capable of being formulated to allow
modulus matching of the adhesive and the substrate.
DETAILED DESCRIPTION OF THE
INVENTION
The 2-cyanoacrylate-based tissue adhesive systems of
the present invention comprise at least one 2-cyanoa-
crylate ester of the general formula (I)
10
15
20
25
30
50
55
60
o CEN (I)
II I
R-O-C—C=CHz
wherein R is selected from the group consisting of alkyl 65
groups having from 1 to about 8 carbon atoms and,
preferably, alkoxyalkyl groups having the formula
R1—O——R2— wherein R1 is an alkyl group having from
4
1 to about 8, preferably 1 to 3 carbon atoms and R2 is an
alkylene group having from 3 to about 6, preferably 3 or
4 carbon atoms, in admixture with from about 2 percent
to about 25 percent, preferably about 5 to 10 percent, of
at least one oxalic acid polymer of the general formula
(11)
o 0
4 II II J7
(R3-o),,—c—c--o H
wherein each R3 is an alkylene group having from 2 to
about 4 carbon atoms, each p is an integer from 1 to
about 4, with the proviso that not more than about 1 of
each 20 p’s is 1, and n is the degree of polymerization
which results in a polymer which does not initiate poly-
merization upon mixing with the 2-cyanoacrylate mon-
omer and standing for about 12 hours. Suitable alkylene
groups include but are not limited to ethylene, propy-
lene, trimethylene, butylene, isobutylene, and tetra-
methylene. It is preferred that p have a value of 3 and
R3 is ethylene. Where p is 1 it is preferred that R3 is
trimethylene.
General methods for the preparation of polyalkylene
oxalates have been described by Shalaby and Jamiolk-
owski (US. Pat. No. 4,140,678). A two step process is
used in which first a monomeric oxalic acid ester such
as diethyl oxalate is transesterified with an alkylene
glycol by heating in an inert atmosphere in the presence
of a catalyst such as sta.nnous octanoate and removing
and collecting the ethanol as it is formed. When the
calculated amount of ethanol has been recovered the
mixture is then heated under reduced pressure to in-
crease the molecular weight of the polymer. The
progress of the reaction may be followed by observing
the infrared hydroxyl peak at 3200-3600 cm“ 1 .
It has been found that the poly(alkylene oxalates) of
U.S. Pat. No. 4,140,678 are not compatible with me-
thoxypropyl 2-cyanoacrylate, a preferred 2-cyanoacry-
late of the class of monomers disclosed in U.S. Pat. No.
3,559,652, even after intensive mixing (sonication) for
24 hours. A more polar oxalate polymer prepared from
triethylene glycol, a polyoxyalkylene glycol, was found
to be completely miscible with the cyanoacrylate mono-
mer. It has been found advantageous to include in the
reaction mixture a small amount (about 5 mol %) of a
low molecular weight, and thus more volatile, glycol
such as trimethylene glycol, which can be stripped
more easily from the reaction mixture to advance the
molecular weight in the second step of the po1yconden-
sation reaction. The infra-red spectra of polymers pre-
pared in this manner did not show evidence that any
signfiicant amount of the glycol was incorporated into
the polymer.
Since the polymer-modified 2-cyanoacrylate compo-
sitions of the invention may polymerize upon heating, to
prepare sterile compositions for clinical use the individ-
ual components should be prepared under sterile condi-
tions and then mixed under sterile conditions and then
placed in sealed microbially impervious containers
made of materials such as polyethylene, polypropylene
and the like which will not initiate premature polymeri-
zation of the composition.
EXPERIMENTAL
Care must be taken at all times to avoid contacting
2-cyanoacrylate-containing materials come with glass,
(11)
5,350,798
5
metals, or water, since polymerization will occur imme-
diately on such contact. All cyanoacrylates were stored
in inert containers under nitrogen at 7° C.
Preparation‘ and Testing of Oxalate Esters
Poly(hexarnethylene oxalate) and poly(trimethylene
oxalate) were prepared according to the method of U.S.
Pat. No. 4,140,678. Samples of each were placed in
sterile 15 ml polypropylene centrifuge tubes with suffi-
cient methoxypropyl 2-cyanoacrylate to form a 10%
wt/vol solution and sealed. After approximately 5 min-
utes mixing the sealed tube was sonicated overnight in a
water bath. No dissolution or mixing of the cyanoacry-
late monomer with the respective polymers was seen.
EXAMPLE 1
Preparation and Testing of Poly[tri(oxyethylene)
oxalate]
A mixture of 26 g (0.20 mol) diethyl oxalate, 31 g
(0.23 mol) triethylene glycol, 0.9 g (0.012 mole) 1,3-pro-
pane diol and 0.0002 mol stannous octanoate as a 0.33M
solution in toluene was placed in a flame-dried polymer-
ization flask equipped with a stirrer and a distillation
head and collection apparatus. All reactants were dis-
tilled or dried in a conventional manner before use. The
flask was heated at 150° C. for 0.3 hour, then 120° C. for
2 hours and then 150° C. for four hours, at which time
the theoretical amount of ethanol had been removed.
The flask then was subjected to a vacuum of less than
0.1 mm Hg and heated to 150° C. for one hour, then
160° C. for three hours, 180° C. for one hour and finally
200° C. for five hours. The progress of the reaction was
monitored by periodically removing a small sample of
the reaction mixture and observing the hydroxyl ab-
sorption peak at 3200-3600 cm"1 of the liquid polymer
spread on a KBr plate. At the end of that time the reac-
tion mixture was allowed to cool and the liquid polymer
was stored in vacuum storage containers at a pressure
below 0.1 mm Hg. On long standing the polymer par-
tially crystallized to a low-melting (about 28° C. as
determined by DSC at 10° C./min heating rate of a
liquid nitrogen quenched sample) solid which reliqui-
fied readily under ambient conditions. The viscosity of
an 0.1% wt/vol solution in chloroform in an Ostwald
viscometer at 31° C. was used to determine the inherent
viscosity '11,-,,;,0.059 (Preparation A).
The compatibility of the polymer with methoxypro-
pyl 2-cyanoacrylate was tested in the same marmer as
the poly(alkylene oxalates) above. Complete miscibility
was observed. In some preparations the molecular
weight was not advanced sufficiently and polymeriza-
tion of the cyanoacrylate occurred on standing. Heating
such polymers for an additional period under vacuum to
increase the molecular weight decreased the hydroxyl
content sufficiently that polymerization no longer oc-
curred when a sample was mixed with monomer in an
dry, inert container. One such preparation yielded poly-
mer having an inherent viscosity '1'),-,,;,0.225 (Preparation
B).
EXAMPLE 2
In Vitro Evaluation of Absorption of Modified
Cyanoacrylates
Samples of pure methoxypropyl 2-cyanoacrylate
(MPC) and poly[tri(oxyethylene) oxalate] modifier
(T OEO) (Example 1, Preparation A) were mixed at 5%
and 10% wt/ vol modifier/cyanoacrylate. 10 ul aliquots
of modified cyanoacrylate and of pure cyanoacrylate
10
15
20
25
30
35
40
45
50
55
60
6
were polymerized in small polyethylene coagulation
cups filled in the presence of a 0.5% aqueous solution of
sodium bicarbonate. The samples were allowed to poly-
merize for at least five minutes and then were trans-
ferred to a large basin filled with the bicarbonate solu-
tion. The samples kept in the bath for an additional 30
minutes to ensure complete polymerization. They were
then removed and rinsed with distilled water, blotted
dry on lint-free absorbent paper and place in a vacuum
desiccator. The samples were from 5 to 8 mm in diame-
ter.
In vitro absorption was studied by measuring weight
loss of samples in a synthetic medium at 5, 15, 25, 50, 75,
100 and 125 days. Test sets of five samples of each com-
position for each test interval were prepared in the
following manner. A phosphate buffer was prepared
using 4.54 g potassium hydrogen phosphate and 14.21 g
sodium dihydrogen phosphate in 2,000 ml distilled wa-
ter. Preweighed 50 ml polypropylene centrifuge tubes
were filled with 50 ml of buffer and a weighed sample of
the modified polymer or homopolymer was placed in
each tube. The tubes were placed in a 37° C. water bath
and agitated for the entire test period.
After each period of time all samples for that period
were evaluated to determine if they had fragmented.
For samples which had not fragmented the buffer solu-
tion was removed and reduced to about 3 ml. The sam-
ple was rinsed with about 50 ml of ultrapure water. The
rinse water was decanted until about 3 ml remained and
the tube was placed in a vacuum desiccator until the
weight of the tube plus sample became constant. The
sample was then removed and the tube was rinsed to
remove any sample fragments and the tube was then
dried in the same marmer. The weight of sample was
then determined by the difference between the weight
of tube plus sample and the weight of the tube. If any
one of a set of samples showed particulate matter all of
the set was treated by a modified procedure, centrifug-
ing at 1,000 rpm for five minutes before decanting the
buffer and after rinsing to ensure capture of all particu-
late matter. The analysis showed that there was a statis-
tically significant difference between the absorbability
of the samples containing 5% modifierand those con-
taining 10%. None of the samples fragmented or broke
up into small pieces, many had the same outward ap-
pearance as at the beginning of the run. This phenome-
non is not unusual, and has been discussed by Kronen-
thal (Polymer Science and Technology, pp.1l3—133, Ple-
num Press, New York 1975). Electron Dispersive Anal-
ysis (EDAX) was carried out to be certain that buffer
residues did not contribute to the sample weights. Only
trace amounts of contaminating species were observed.
The data were treated statistically using the General
Linear Models Procedure of SAS Institute and are pres-
ented in Table 1.
TABLEI
Days
5 15 25 50 75 100 150
%loss — — 5.77 12.26 12.14 7.82 8.97
MPC
» %loss 13.36 35.74 51.12 74.53 37.34 93.15 94.83
5% TOEO
%loss 24.85 32.40 47.84 45.24 57.87 53.02 56.07
10% TOEO
65
The adhesive performance of isobutyl cyanoacrylate
(IBC), methoxypropyl cyanoacrylate (MPC) and MPC
5,350,798
7
containing 10% poly[(trioxyethylene) oxalate] (TOEO)
from Example 1, Preparation B were compared in vitro
using goat skin as the substrate.
After closely clipping the hair, skin was harvested
from the sides of a goat carcass as three marked and
measured 3" by 12" strips. The skin had been frozen for
eleven days and was removed from the carcass on the
twelfth day. The skin patches were pinned to pans
which had been coated with paraffin to simplify pinning
and covered with normal saline and stored in a refriger-
ator overnight. The following day the saline was
drained and excess moisture blotted off with lint-free
paper. A 28 mm incision was made down the middle of
each patch along the long axis with a #10 scalpel, being
certain not to sever the skin at either end of the cut.
Taking care that the skin was in its original cut size, the
cross-sectional areas of the incisions were determined
by measuring across the cut site and multiplying by the
width of each of the sample cuts (2.5 cm). The incision
was dabbed with a gauze sponge to remove excess mois-
ture and a 1 ml aliquot of the test adhesive was intro-
duced along the incision line. The incision was quickly
opposed using finger pressure to hold the sides together
for about 30 seconds. The samples were allowed to
polymerize for at least 3§hours. The IBC polymerized
the most rapidly, the MPC more slowly, and the modi-
fied adhesive slightly more slowly than the MPC. The
IBC formed a stiff crust at the incision site as has been
reported in the literature, but neither the MPC nor the
modified polymer appeared to do so. Then Zlcm strips
were cut transversely to the incision line by first plung-
ing the scalpel into the glued incision and then making
a standard cut from that point to complete the sample.
Samples were then tested on an Instron at a stroke rate
of 0.6 in/sec, using a 500 lb load cell at 10% of full
range. The results are shown below.
IBC 19 samples 31.25 i 5.99 psi (95% conf. level)
MPC 18 samples 33.47 1‘ 6.25 psi "
10% TOEO/MPC 19 43.86 i 7.05 psi "
samples
It is clear that the tensile strength of the adhesive made
with the modified polymer is significantly greater than
either of the homo polymers, which in turn showed no
significant difference between one another.
Tests were also made joining bone, where MPC and
the composition of the present invention were both
observed to be less effective than IBC.
EXAMPLE 4
Cytotoxicity testing was performed on IBC, MPC
and MPC containing 10% TOEO (Example 1, Prepara-
tion B) using a modification of ASTM F 895-84 “Stan-
dard Test Method for Agar Diffusion Cell Culture for
Cytotoxicity”. The actual test method was changed by
placing 10 pl aliquots of MPC and 10% TOEO/MPC
monomers directly on confluent monolayers of mouse
fibroblasts from which most of the culture medium had
been removed, rather than on an agar diffusion barrier.
The negative control was medium and a monolayer to
which no additive was introduced. The positive control
was a copper penny placed directly on the center of the
5
10
15
20
25
30
35
40
45
50
55
60
65
8
monolayer. The monomers were allowed to polymerize
for at least one minute and then 2.0 ml of the culture
medium was pipetted back into the 35 mm culture plate.
The plates were incubated for at least 24 hours and then
qualitatively evaluated. The results were taken from
photographs taken at 200x magnification about 1 cm
from the polymerized sample or penny, and the middle
of the plate of the negative control. The negative con-
trol showed a crowded confluent monolayer. The posi-
tive control showed signs of total cellular lysis, only
cellular debris being observed. The plates of the MPC
and the 10% TOEO/MPC both appeared to have
clumping of detached cells with little or no discernible
lysis. The MPC plate appeared to have some cells that
were just in the process of detaching. It is unclear
whether the reaction observed was the result of fast-
absorbing monomeric byproducts causing cellular de-
tachment or was due to a toxic response associated with
the high initial dose of monomeric adhesive which was
used to ensure a response within a short time period.
We claim:
1. A composition of matter which comprises at least
one 2-cyanoacrylate ester of the general formula (I)
0 CEN
H I
R—O—C—C::CHz
(1)
wherein R is selected from the group consisting of alkyl
groups having from 1 to about 8 carbon atoms and
alkoxyalkyl groups having the formula R1-O—R2—
wherein R1 is an alkyl group having from 1 to about 8
carbon atoms and R2 is an alkylene group having from
3 to about 6 carbon atoms, in admixture with from about
2 percent to about 25 percent of at least one oxalic acid
polymer of the general formula (II)
o 0 (11)
II 11
{.01-.—o),.-—c—c—o ..
wherein each R3 is an alkylene group having from 2 to
about 4 carbon atoms, each p is an integer from 1 to
about 4, with the proviso that not more than about 1 of
each 20 p’s is 1, and n is the degree of polymerization
which results in a polymer which does not initiate poly-
merization upon mixing with the 2-cyanoacrylate
monomer and standing for about 12 hours.
2. The composition of claim 1 wherein the 2-cyanoa-
crylate is 2-methoxypropyl 2-cyanoacrylate.
3. The composition of claim 1 wherein the oxalic acid
polymer is a copolymer of oxalic acid with triethylene
glycol and a minor amount of 1,3-propanediol.
4. A composition of matter which comprises 2-
methoxypropyl 2-cyanoacrylate and a copolymer of
oxalic acid with triethylene glycol.
5. The composition of claim 4 which further com-
prises a minor amount of 1,3—propanediol in the oxalate
copolymer.
6. The cured composition of claim 1.
7. The cured composition of claim 4.
8. A sterile preparation which comprises the compo-
sition of claim 1.
* * * * *
Coments go here:
- Log in to post comments