Fluorocyanoacrylates
Folder:
Year:
Abstract:
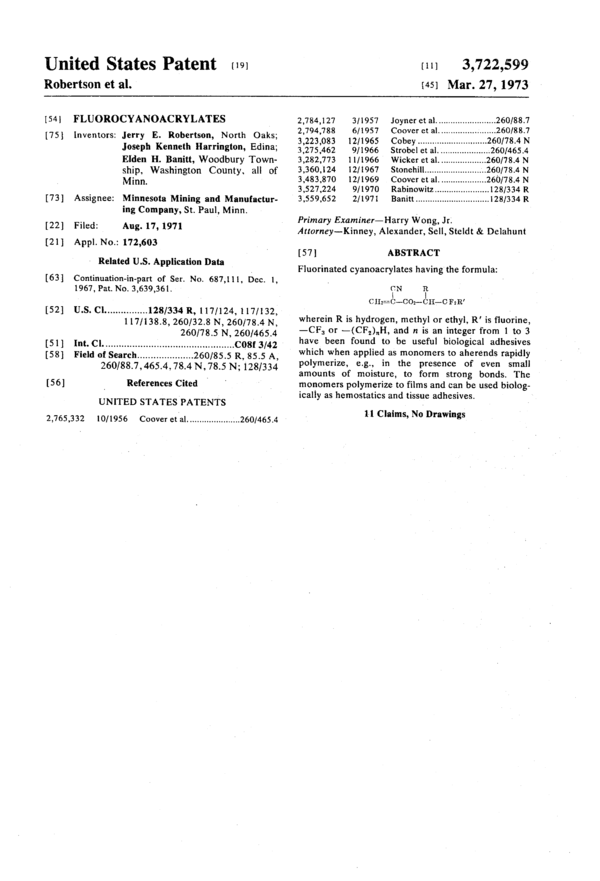
Fuorinated cyanoacrylates having the formula:
-------
wherein R is hydrogen, methyl or ethyl, R' is fluorine -CF3 or -(CF2)nH, and n is an integer from 1 to 3 have been found to be useful biological adhesives which when applied as monomers to aherends rapidly polymerize, e.g., in the presence of even small amounts of moisture, to form strong bonds. The monomers polymerize to films and can be used biologically as hemostatics and tissue adhesives.
Type of document:
Language:
United States Patent [19]
Robertson et al.
[11] 3,722,599
[451 Mar. 27, 1973
[541 FLUOROCYANOACRYLATES
[75] Inventors: Jerry E. Robertson, North Oaks;
Joseph Kenneth Harrington, Edina;
Elden H. Banitt, Woodbury Town-
ship, Washington County. all of
Minn.
[73] Assignee: Minnesota Mining and Manufactur-
ing Company, St. Paul, Minn.
[22] Filed: Aug. 17,1971
[21] Appl.No.: 172,603
Related U.S. Application Data
[63] Continuation-in-part of Ser. No. 687,111, Dec. 1
1967, Pat. No. 3,639,361.
[52] U.S. Cl .............. ..128/334 R, 117/124, 117/132,
117/138.8, 260/32.8 N, 260/78.4 N,
260/78.5 N, 260/465.4
[51] Int. Cl ............................................... ..C08f 3/42
[58] Field of Search ................... ..260/85.5 R, 85.5 A,
260/88.7,465.4, 78.4 N, 78.5 N; 128/334
[56] _ References Cited ’
UNITED STATES PATENTS
2,765,332 10/1956
Coover et al. ................... ..260/465.4
2,784,127 3/1957 Joyner et al. ...................... ..260/88.7
2,794,788 6/1957 Coover et al. ..... ..260/88.7
3,223,083 12/1965 Cobey .......... .. ...260/78.4 N
3,275,462 9/1966 Strobel et al. ....260/465.4
3,282,773 11/1966 Wicker et a1. 260/78.4 N
3,360,124 12/1967 Stonehill ........ .. 260/78.4 N
3,483,870 12/1969 Coover et al. ..260/78.4 N
3,527,224 9/1970 Rabinowitz..... ....128/334 R
3,559,652 2/1971 Banitt ............................. ..128/334 R
Primary Examiner—Harry Wong, Jr.
Attorney-Kinney, Alexander, Sell, Steldt & Delahunt
[57] ABSTRACT
Fluorinated cyanoacrylates having the formula:
‘EN ‘F
CIIz=C—-CO2——CH—C F2R’
wherein R is hydrogen, methyl or ethyl, R’ is fluorine,
—CF3 or —(CF2),,H, and n is an integer from 1 to 3
have been found to be useful biological adhesives
which when applied as monomers to aherends rapidly
polymerize, e.g., in the presence of even small
amounts of moisture, to form strong bonds. The
monomers polymerize to films and can be used biolog-
ically as hemostatics and tissue adhesives.
1-1 Claims, No Drawings
3,722,599
1
FLUOROCYANOACRYLATES
This application is a continuation-in-part, divided
from our copending application Ser. No. 687,111 filed
Dec. 1, 1967, issued as U. S. Pat. No. 3,639,361 on
Feb. 1, 1972.
This invention relates to the method of adhering tis-
sues and effecting hemostasis by polymerizing fluoro-
cyanoacrylates to form coatings and adhesives. More
particularly, the invention relates to the process for
hemostasis and biological adhesion by fluorine-con-
taining cyanoacrylate monomers which polymerize
rapidly upon contact with basic materials, e.g.,
moisture in the air, to form polymers which coat living
mammalian tissues and of which two such coated sur-
faces are coherent and adhere.
Known non-fluorinated alkyl 2-cyanoacrylates, par-
ticularly the methyl, isobutyl and n-butyl 2-cyanoacry-
lates, have been investigated for use as biological adhe-
sives, see, e.g. Medical World News, 8 (20), 41 (1967);
Mfg. Chemist, 38 (8), 94 (1967); Technical Report
6618, Walter Reed Army Medical Center, December,
1966‘. While the unsubstituted alkyl monomers appear
to possess the requisite bonding and hemostatic proper-
ties when applied to damaged mammalian tissues, these
materials appear to fail to have the required properties
of low toxicity and adequate resorption or absorption
by the tissues. Methyl 2-cyanoacrylate, for example,
gives rise to a severe inflammatory tissue response at
the site of application. The n-butyl and isobutyl 2-
cyanoacrylate monomers are not absorbed well (if at
all) by the tissues and polymeric residue of the adhesive
has been observed by histologic examination of the site
of application as much as 12 months after surgery, see,
e.g. Medical World News, 8 (29), 27 (1967).
Fluorinated cyanoacrylates have been suggested in
U.S. Pat. No. 3,255,059 as possible components, i.e.,
binders, of polymeric rocket propellant compositions.
No workable synthetic method is provided for the
monomers. No suggestion of the advantages of
fluorinated 2-cyanoacrylates for biological applications
is made.
It was surprising and unexpected to find that the ad-
hesives of the present invention are readily assimilated
by the body with minimal toxic effects although the
methods by which the body accomplishes this and in-
termediate and final products formed are at present
unknown.
It is an object of this invention to provide a novel
method for effecting biological adhesion and hemosta-
sis. Desirably, the method is effective in the presence of
blood and other body fluids and natural healing is not
impaired. Other objects will become apparent
hereinafter.
It has been found that fluorinated 2-cyanoacrylates
are useful in adhesive compositions and are particularly
useful as biological adhesives and hemostatic agents.
Thus, the present invention provides new methods for
the joining together, adhesive suturing of mammalian
tissues, as well as for arresting the escape of blood
therethrough. For example, satisfactory hemostasis of
splenic and liver wounds, heretofore to a great extent
unrepairable by conventional methods, is obtained by
the application and polymerization of a thin film of
fluorinated 2-cyanoacrylate monomer on the injured
surface. The bonding strength, absorbability by the tis-
sues, hemostatic capability and low degree of local in-
10
15
20
25'
30
35
40
45
50
55
60
65
2
flammation resulting when these monomers are applied
in vivo, are properties which make them especially
valuable for biological applications.
The present invention also contemplates using
fluorocyanoacrylates alone, or in conjunction with
each other or in conjunction with unsubstituted alkyl 2-
cyanoacrylates in the bonding of damaged mammalian
tissue or in preventing the escape of blood or other
fluids therethrough. Comonomer compositions are of
interest for specific uses because they may provide ad-
vantageous combinations of properties not completely
embodied in individual monomers.
The process of the present invention is possible as a
result of the discovery that the replacement of
hydrogen atoms in the alcoholic residue of 2-
cyanoacrylate esters with fluorine atoms unexpectedly
renders these monomers substantially better tolerated
by living tissue than are the non-fluorinated hydrocar-
bon monomers, and provides compounds which are
surprisingly more readily biodegraded or absorbed.
The monomeric 2-cyanoacrylate esters which are
employed in the process of this invention are
represented by the structural formula:
CN I11.
CH2=$—CO2—CH—C F2R’
wherein R is a member of the group consisting of
hydrogen, methyl or ethyl and R’ is a member of the
group consisting of fluorine, —(CF,),,l-l and —CF3 and
n is an integer from 1 to 3. These new fluorinated
monomers when employed in the process of the inven-
tion as biological adhesives or hemostats, individually
or as comonomers, exhibit excellent wound adhesion
and hemostasis; they are well assimilated by the tissues
at an acceptable rate, and their use, particularly in the
cases of the monomers in which the
R
—(£H—C FzR’
radical is one of —CH2(CF,).H, —CH,CF,CF3, —
CH2(CF2)2l-I and —CH(CH3)CF3 produces minimal
local tissue inflammation in mammals. The —CH,CF,,
group-containing monomer, 2,2,2-trifluoroethyl
cyanoacrylate, while exhibiting a relatively higher
degree of inflammatory tissue response in subcutane-
ous tissues of mice, was completely biodegraded by the
exposed surface of partially excised rat livers within 16
weeks after application of the monomer. The degree of
local inflammation caused by the latter monomer, how-
ever, is still less than that caused by methyl 2-
cyanoacrylate as determined by gross and microscopic
examinations.
In most bonding applications employing monomers
of the invention, polymerization is catalyzed by small
amounts of moisture on the surface of the adherends,
thus desired bonding of tissues or hemostasis proceeds
well in the presence of blood and other body fluids. The
bonds formed are of adequate flexibility and strength to
withstand normal movement of tissue. In addition,
bond strength is maintained as natural wound healing
proceeds concurrently with polymer assimilation.
Compositions employed in the invention are
sterilizable by conventional methods such as distillation
under aseptic conditions. _
The method of the invention for repairing injured tis-
3,722,599
3
sues (for example, to control bleeding) comprises, in
general, sponging to remove superficial body fluids and
subsequent application to the exposed tissue of an ad-
hesive composition containing a fluorocyanoacrylate
monomer of the group which composition polymerizes
to a thin film of polymer while in contact with the tissue
surface. Tissues which are not bleeding or otherwise
covered by body fluids need not be sponged first. For
bonding separate surfaces of body tissues, the
monomer is applied to at least one surface, and the sur-
faces are brought quickly together while the monomer
polymerizes in contact with both of the surfaces.
The process of the invention is particularly useful for
dental applications such as controlling the bleeding fol-
lowing extractions, bleeding accompanying prophylaxis
or restorations, and bleeding due to gingevectomy and
other periodontal treatments.
The process of the invention employs adhesive for-
mulations in which fluoroalkyl 2-cyanoacrylates are the
major active constituent suitably combined in admix-
ture with a polymerization inhibitor such as sulfur diox-
ide. One or more adjuvant substances, such as thicken-
ing agents, plasticizers, or the like, to improve the sur-
gical utility of the monomer, can also be present.
Depending on the particular requirements of the
user, these adhesive compositions can be applied by
known means such as with a glass stirring rod, sterile
brush or medicine dropper; however, in many situa-
tions a pressurized aerosol dispensing package is
preferred in which the adhesive composition is in solu-
tion with a compatible anhydrous propellant. Aerosol
application of the monomers is particularly ad-
vantageous for use in hemostasis.
The monomers are readily polymerized to addition-
type polymers and copolymers, which are generally op-
tically clear (as films) having the general formula:
—l:-—CH2—§N -1
l
002
Ht-..
?F2
R’ .
where R and R’ are as defined above and x is a number
from 5 to 500.
The preferred method for preparing high purity (95
percent or greater) fluoroalkyl 2-cyanoacrylates com-
prises catalyzing the condensation of formaldehyde
with esters of cyanoacetic acid by means of a mixture
of an acid and the acidic salt of an alkyl primary or
secondary amine.
This method is described in detail in the parent U.S.
Pat. Application Ser. No. 687,1 ll and corresponding
foreign applications and patents, e.g., Netherlands Ser.
No. 68/17168 and Great Britain Pat. No. 1,21 l,l72.
The following examples will illustrate preferred em-
bodiments of the invention. It will be understood, how-
ever, that the examples are included merely for the pur-
poses of illustration and not intended to limit the scope
of the invention, unless otherwise specifically in-
dicated. All parts are by weight unless otherwise
specified, and the pressures are shown in millimeters of ,
mercury.
EXAMPLE 1
Discs of polymer are made by injecting test monomer
l0
15
20
25
30
35
40
45
50
55
60
65
4
subcutaneously in female rats and allowing it to
polymerize. The chunks of polymer are recovered ap-
proximately 24 hours later, washed, vacuum dried,
weighed and then implanted subcutaneously in the dor-
sal neck tissue ofa second group of mice. Stainless steel
wound clips are used to close the skin incisions.
Animals are sacrificed at 2, 4, 8 and i6 weeks, and the
material is recovered, washed, dried and reweighed
then reimplanted. Local and systemic gross tissue reac-
tions and adhesions are noted at necropsy. Absorption
of the test material is determined on the basis of weight
loss.
The compounds enumerated in Table l were evalu-
ated using this general method.
TABLE 1
Compound
I) l, l ,5-trihydroperfluoro-n-
Absorption at 16 weeks
- 35% (average of three
pentyl 2-cyanoacrylate different lots)
ll) 1,1,1-trifluoroisopropyl [796 (average of five
2-cyanoacrylate different lots)
lll) 2,2,2-trifluoroethyl 100%
2-cyanoacrylate
lV) l,l-dihydroperfluoro—n-
propyl 2-cyanoacrylate
V) 1,1,3-trihydroperfluoro-m
propyl 2—cyanoacrylate
100% (2 weeks)
100% (2 weeks)
EXAMPLE 2
Hemostasis in Vascular Organs (Excised Cat Spleen)
A male cat was anesthetized intravenously with pen-
tobarbital-sodium and prepared for aseptic surgery.-
Prior to surgery, 25 milligrams of heparin sodium USP
were intravenously administered. The spleen was ex-
teriorized through a ventral midline incision, and a
disk-shaped portion of splenic tissue 1 to 2 centimeters
in diameter and 3 to 5 millimeters deep was excised.
Resulting profuse hemorrhage from the wound was
controlled by occluding the blood supply to the spleen
with soft clamps and gauze sponging. A thin layer of
2,2,2-trifluoroethyl 2-cyanoacrylate adhesive
monomer was applied to the wound surface immediate-
ly thereafter by spraying with an aerosol at a distance of
from 4 to 8 centimeters from the wound surface. After
allowing sufficient time for polymerization of the
monomer, the organ was replaced in the peritoneal
cavity. The ventral midline incision was closed using
conventional sutures.
The cat, except for depression during the first few
post-operative days, made an uneventful recovery and
remained healthy until sacrificed 6 weeks after surgery.
Adhesions between the spleen and surrounding tissue,
an expected sequela following surgery of this nature,
and mild inflammation of the splenic capsule were the
only gross tissue changes observed at necropsy. None
of the adhesive was grossly visible and normal healing
appeared to be in progress.
In a similar manner, other compounds as designated
in Example 1 were used for hemostasis of excised
female rat spleen as shown in Table 2.
TABLE 2
Method of
Compound Application Hemostasis Tissue Irritation
l liquid good none
ll liquid excellent very little
&722§99
5
EXAMPLE3
Hemostasis in Vascular Organs (Excised Rat Liver)
The liver of an anesthetized animal is exteriorized
and the distal one-fourth to one-third of the left lateral
lobe is excised. Hemorrhage is controlled by digital
compression while a thin coating of test material is ap-
plied in either liquid or aerosol form. After allowing
sufficient time for polymerization, the digital pressure
is released. The liver is replaced in the peritoneal cavity
and the muscle and skin wounds are closed with con-
ventional sutures. Adhesive handling and hemostatic
properties are recorded at the time of application. Ab-
sorption rates of the test materials are noted at
necropsy 2, 4 and 8 weeks following liquid application
and 1, 2 and 4 or more weeks after spraying.
The compounds (as designated in Example 1) tabu-
lated in Table 3 were evaluated using this general
method.
TABLE 3
Form
Compound Applied Hemostasis Estimated Absorption
Ill aerosol good 1) 100% at 16 weeks
IV liquid 1) good 1) 100% at 4 weeks
2) poor 2) 100% at 2 weeks
V liquid 1) fair ' 1) 100% at 8 weeks
2) good 2) 100% at 2 weeks
ll & Ill liquid good slightly at 8 weeks
Copolymer
(50/50)
EXAMPLE 4
Skin Incision Test
Single midline skin incisions are made in the dorsal
neck region of anesthetized rats. Blood is allowed to
flow before sponging the wound with gauze. The test
material is applied and spread as a thin coating along
the wound edge. Immediately following adhesive appli-
cation, the wound edges are apposed using digital pres-
sure and tissue forceps. After allowing sufficient time
for polymerization, the forceps are released. Each
animal is postoperatively observed for general condi-
tion and the wounds are scored at 24 hours using the
following code:
OPEN-POOR (OP) wound edges open -
poorly aligned
OPEN-FAIR (OF) wound partially open —
fair alignment '
CLOSED-FAIR (CF) wound closed — fair
alignment
CLOSED-GOOD (CG) wound completely
closed— good alignment
Following 24 hours wound scoring, the representa-
tive closed-good animals and, in some cases, closed-fair
animals are sacrificed and the center section of the
wound, 3 cm. long by 2 cm. wide, is dissected free. One
side of the wound is placed in a fixed clamp and the
other side in a clamp attached to a suspended plastic
container. Water is allowed to flow into the container
at a constant rate until the wound separates. The
weight of the container plus the added water deter-
mines wound tensile strength measured as grams to
give separation. Compounds as designated in Example
1 were tested by this method with the results set forth in
Table 4.
10
15
20
25
30
35
40
45
50
55
60
65
6
TABLE4
24 Hour Wound Average Wound Tensile
Compound Appearance Strength in Grams
1 CG 10/10 664
11 CG 10/10 575
Ill CG 8/10
CF 1/10 426
OF 1/10
IV CF 4/I0
CF 1/10
CF 3/10 314
01’ 2/10
V CG 6/10 326
CF 1/10 241
01’ 3/10
ll&lll CG 10/10 389 -
Copolymer
(50/50)
EXAMPLE 5
Hemostasis in Oral Surgery
Male Rhesus monkeys were anesthetized with a 2
percent solution of thiamylal (intravenous) and four
canine teeth of each monkey were extracted. The
hemorrhaging of the tooth socket was controlled by ap-
plication of 1,1,1-trifluoroisopropyl 2-cyanoacrylate
spray. The aerosol spray composition was 10.6 percent
monomer (by weight) and the propellant was 30 "per-
cent dichlorodifluoromethane, 25 percent
trichloromonofluoromethane and 45 percent 1,2-
dichlorotetrafluoroethane. After 5 days, all monkeys
had recovered and were able to eat their normal diet.
Healing was complete, with all sockets filled in, 1 -
month after surgery.
What is claimed is:
l. A process for the repair of living tissue which com-
prises applying to a surface of said tissue a composition
comprising a monomer represented by the formula:
CN R
CHz=(|3—C0z———(J)H—C F2R'
wherein R is a member of the group consisting of
hydrogen, methyl or ethyl, R’ is a member of the group
consisting of fluorine,’—CH3 and —(CF2),.I-I and n is an
integer from 1 to 3 and permitting polymerization
thereof in contact with said surface.
2.Tl1é process for the repair of living tissue accord-
ing to claim 1 comprising the additional step of at least
partially freeing the surface from superficial body fluids’
before application of monomer containing composi-
tion.
3. The process for the repair of living tissue accord-
ing to claim 1 comprising the additional step of appos-
ing the surface to which monomer is first applied to a
second surface of tissue and maintaining contact while
polymerization of the monomer proceeds.
4. The process for the repair of living tissue accord-
ing to claim 3 wherein monomer is additionally applied
to the second surface before first and second surfaces
are apposed.
5. The process of claim 1 wherein the living tissue is a
vascular organ.
6. The process according to claim 1 wherein the
monomer is applied in finely divided form as a spray
until a coating of a desired amount is present on the tis-
sue being repaired.
7. The process according to claim 6 wherein the tis-
sue being repaired is a vascular organ.
3,722,599. l
7 8
8. The process according to claim 6 wherein oral tis- monomer is 1,1,1-trifluoroisopropyl 2-cyanoacrylate.
sue is repaired. 11. The process according to claim 5 wherein the
9. The process according to claim 1 wherein the monomer is l,l,l-trifluoroisopropyl 2-cyanoacrylate.
monomer is 1,1,1-trifluoroisopropyl 2-cyanoacrylate.
10. The process according ‘to claim 3 wherein the 5
10
15
20
25
30
35
40
45
50
55
60
65
Coments go here:
- Log in to post comments