Cyanoacrylate Adhesive Composition
Folder:
Year:
Abstract:
A rapid setting .alpha.-cyanoacrylate-based adhesive composition having an improved stabilizer therein. The stabilizer is a sulfamide derivative containing the group
----
Stabilizing may be further improved by using the sulfamides in combination with phenolic anti-oxidants, particularly sterically hindered phenols, without reducing the curing rate of the composition.
Type of document:
Language:
United States Patent 119]
Reich et al.
[11] 4,414,347
» [45] Nov. 8, 1983
[54] CY ANOACRYLATE ADHESIVE
COMPOSITION OTHER PUBLICATIONS
[75] Inventors: Karl Reich, Carlsberg; Heinz Sieger, fa’ R" Chemical Abstracts’ Vol’ 57’ 14932g
gppelheim both of Fed Rep‘ of Appel. Rolf and Gerber Hermann- Chem Ber 91 pp
Emmy . 1200-1203 (1958).
[73] Assignee: Teroson GmbH, Heidelberg, Fed. _ _ _
Rep_ of Germany Primary Exammer—\_/eronlca P. Hoke I
Attorney, Agent, or F1rm——Howard J. Troffkm
[21] Appl. No.: 418,496
_ 57] ABSTRACT
[22] Filed: Sep. 15, 1982 _ _ .
A rap1d setting a-cyanoacrylate-based adheslve compo-
[51] Int. CL3 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. C08K 5/ 43 sition having an improved Stabilizer therein The stabi_
12:1 a
560/148;564/79;526/298 O O
[56] References‘Cited _g_NH_S02_NH_g_.
ILSPATENTDOCUMENTS
2,765,332 10/1956 COOVCI‘ et 31. .................... .. 526/298 Stabilizing may be further impfoved by using the sulfa-
2,855,375 10/1958 Dobay ................ .. 524/169 mides in combination with phenolic anti_oxidants pap
3,454,606 7/1969 Brotherton et al. . 560/148 ticularl steri H h- d d h 1 - h d’ -
3,455,892 7/1969 Froelich .................. .. 564/79 ?' °a 3’ m "6 P. ‘?“° 5’ W” °“‘ ‘C “mg
3,527,841 9/1970 Wicker et a1. .. 526/298 the °f“““8 Tate 0f the .°°mP°S1“°n- '
3,856,786 12/1974 Huber ............. .. 560/148
3,915,931 10/1975 Gilleo et a1. ...................... .. 524/168
viscosity
(in Pa.s)
400
300
200
100
5 Cleims, 1 Drawing Figure
20
Storage time 1: (days) at 50°C
U.S. Patent Nov. 8, 1983 4,414,347
Figure 1
Viscosity
(m Pa.s)
1
400
300
2
3
200 4
100
O
20
Storage time t (days) at 50°C
4,414,347
1
CY ANOACRYLATE ADHESIVE COMPOSITION
BACKGROUND OF INVENTION
The present invention relates to a a-cyanoacrylate-
based adhesive composition having good storage stabil-
ity.
a-eyanoacrylates of general formula
CN
/
1-[2C=C
COOR
are rapid-setting adhesives which are ideally suited for
the adhesion of a large number of materials like rubber,
plastics, metal, leather and wood in various technical
areas like machine construction, apparatus construc-
tion, electrical engineering, jewelery industry, watch-
maker industry, household etc. The parts can be joined
in an extremely short time of only a few seconds and the
adhesive joints produced in this way have good me-
chanical strength characteristics. The reactive mono-
mers forming the one-component adhesive are hard-
ened mostly in the absence of a catalyst by anionic
polymerization initiated by even small traces of ex-
tremely weak basic-acting compounds (Lewis bases)
such as, for example, water (e. g. surface moisture) when
applied as a thin film between the parts to be joined.
Due to the extreme reactivity of these adhesives usu-
ally inhibition of premature polymerisation which has
an adverse effect on storage stability is required. Ac-
cordingly in the past acidic gases as sulphur dioxide,
nitrogen monoxide, carbon dioxide, hydrogen fluoride
etc. have been added to these adhesives. Furthermore
proton acids including mineral acids like hydrochloric
acid, sulphuric acid and polyphosphoric acid, anhy-
drides like phosphoric anhydride, sulfonic acids and
carboxylic acids like acetic acid, itaconic acid and ben-
zenesulfonic acid have been usedas stabilizers. Typical
patents relating to the aforementioned stabilizers are
U.S. Pat. Nos. 2,765,332, 2,794,788, 2,756,251,
2,912,454, 2,926,188, 3,728,375, 3,993,678 and DE-OS
No. 23 O7 834.
However, gaseous stabilizers are only suited to a
limited degree for effective stabilization of a-cyanoa-
crylates since metering in the liquid adhesive is difficult
and during storage undefined amounts can prematurely
escape. When using higher concentrations of acidic
gases or non-gaseous acids the curing rate of the CL-
cyanoacrylates rapidly decreases. On the other hand
when using too low Concentrations no sufficient stabili-
zation is achieved.
It has now been surprisingly found that the above
difficulties and disadvantages of the prior art stabilizers
can be obviated by the use of certain compounds de-
scribed herein below as stabilizers contained in a-
cyanoacrylate-based adhesive compositions. These
compounds can be easily added in well defined amounts
in form of solid or liquid substances, require only lim-
ited concentrations in the resulting adhesive composi-
tions based on a-cyanoacrylate and lead to improved
storage stability. Furthermore the efficiency of conven-
tional stabilizers is greatly enhanced when combined
with these compounds.
10
U
20
25
30
35
40
45
50
55
60
65
2
DETAILED DESCRIPTION OF INVENTION
The present invention is directed to adhesive compo-
sitions based on a-cyanoacrylate and which contain a
stabilizer and may contain conventional additives as
described herein and in the appended claims.
The generally known a-cyanoacrylates which serve
as a basis for the adhesive compositions according to the
invention are of general formula:
CN
/
I'I2C=C
COOR
wherein R is straight or branched-chain alkyl group
such as methyl, ethyl, n-propyl, isopropyl, n-butyl, iso-
butyl, pentyl, hexyl, and the like as well as a halogen
atom or alkoxy group substitued alkyl such as 2-chloro-
ethyl, 3-chloropropyl, 2-chlorobutyl, trifluoroethyl,
2-methoxyethyl, 3-methoxybutyl, 2-ethoxyethyl group
and the like; a straight or branched-chain alkenyl group
such as allyl, methallyl, crotyl and the like; a straight or
branched-chain alkinyl group such as propargyl and the
like; cycloalkyl group such as cyclohexyl, and the like;
an ether group X—O—Y or X———O——X—O—Y in
which X is a saturated alkylene group having 2 to 4
carbon atoms and Y is an alkyl group having 2 to 6
carbon atoms; an aryl group such as phenyl and the like;
or an aralkyl group, such as benzyl, cresyl and the like..
Further, German published application DE-OS No.
2,816,836 refers to a large number of suitable a-cyanoa-
crylates which are incorporated herein by reference.
The adhesive compositions according to the invention
may, in addition, contain conventional additives such as
thickeners, plastisizers, polymerization catalysts, fur-
ther copolymerizable monomers, solvents, perfumes,
dyes, pigments, etc. Of course, the adhesive composi-
tions according to the invention may additionally con-
tain conventional polymerization inhibitors. These ad-
ditives are conventionally known, form part of the prior
art and examples of them are described in the herein-
above mentioned references. Based on the total weight
of the adhesive compositions according to the invention
those additives are usually present in an amount of less
than 10%.
The subject stabilizers contained in the adhesive com-
positions according to the invention are sulfamide deri-
vates containing the group
0 0
II II
—C—NH-SO2—NH—C--.
Sulfamide derivatives used according to the invention
are preferably compounds of the following general
formula
0 0 I
II II
R‘—C—NH—SO2—NH-C-R1
In this formula, the radicals R1 are the same or differ-
ent and in each case stand for hydrogen or for linear or
branched-chain alkyl with 1 to 18 carbon atoms, cyclo-
alkyl with 3 to 8 carbon atoms, phenylmethyl or alkoxy
—-—OR2, where R2 is a linear or branched-chain alkyl
with 1 to 18 carbon atoms, cycloalkyl with 3 to 8 carbon
4,414,347
3
atoms, phenyl, phenylalkyl with 1 to 4 carbon atoms in
the alkyl group, or alkylphenyl with 1 to 4 carbon atoms
in the alkyl group or groups. R1 can also be trifluoro- or
trichloromethyl or alkoxy —OR2, wherein R2 is diphe-
nyl- or triphenylmethyl.
N,N’-diacyl-sulfamides have long been known. Thus,
C. H. Anderson and E. F. Degering describe in Proc.
Indiana Acad. Sci. 1946, 56, 134-135 the preparation of
certain representatives of this class of compounds with
a yield of 55% of theory by acylating sulfamide in the
absence of solvents in accordance with the following
equation:
if E?
ZCH3--C—0—C-CH3 + NH;-S02--NH; ——-9
o 0
[I ll
CH3—C-NH—-SO;-NH--C—CH3 + 2CH3COO1-I
German Pat. No. 876,846 describes a modified process
which is also based on the acylation of sulfamide. A
yield of 78% of theory is given therein for the prepara-
tion of N,N’-diacetyl sulfamide.
As the preparation of sulfamide is a time-consuming
and complicated process (cf HOUBEN-WEYL, Meth-
oden der Organischen Chemie, Vol. XI/2, 4th edition,
1958, 713) and provides sulfamide with only 44% yield,
it was of interest to develop a process for supplying the
desired N,N’-diacyl sulfamides with high yield and
purity.
It has surprisingly been found that in sulfuryl diisocy-
anate OCN—SO2—NCO both isocyanate groups react
smoothly with carboxylic acid providing N,N’-diacyl
sulfamides in high yields:
0
II
2R--C-OH + OCN—SO2—NCO ——%
o o
II II
R—-C—HN—SO2—NH-C—R + zcoz
It is merely necessary to heat the two components in an
organic solvent until CO2 evolution stops. This gener-
ally requires at a temperature of 70° to 80° C. a time of
1 to 2 hours. The sulfuryl diisocyanate required as the
starting substance can be obtained by the process of
German Pat. No. 940,351 (of also HOUBEN-WEYL,
Methoden der Organischen Chemie, V01. XI/2, 4th
edition, 1958, 724).
With particular advantage, the reactants used are
aliphatic carboxylic acids and R can be hydrogen, a
linear or branched-chain C1—C1g alkyl group or a
C3-C3 alkyl group or a benzyl group.
The above process has made it possible to prepare for
the first time N,N’-diformyl sulfamide and N,N’-bis-(cy-
clohexylcarbonyl)-sulfamide, neither of which are de-
scribed in the literature.
Through the selection of a suitable solvent in which
the starting substances are soluble but the desired end
product is insoluble, the latter slowly crystallizes and is
obtained with a high purity and does not have to be
recrystallized.
It has proved advantageous for certain uses to per-
form the reaction in a solvent in which the end product
is also soluble. This is, for example, the case if N,N’-dia-
cyl sulfamides are to be further processed in the form of
a solution, so that it is unnecessary to redissolve the
10
15
20
25
30
35
40
45
50
55
60
65
4
reaction products. According to a specific embodiment
of the invention, tetrahydrofuran is used as a solvent,
because the latter participates in the reaction through
polymerization giving stabilizers which are not precipi-
tated from the solution during dilution with inert sol-
vents such as benzene, and this greatly facilitates the use
according to the invention as stabilizer in a-cyanoacry-
late-based adhesives.
N,N’-bis-(alkoxycarbonyl)-sulfamides and N,N’-bis-
(aryloxycarbonyl)-sulfamides can be obtained in quanti-
tative yield by adding the corresponding alcohols or
phenols to sulfuryl diisocyanate:
2R—oH + OCN-SO2—NCO —%
o o
I! ll
R0-C—NI-1-S02-NH-C—OR
cf U.S. Pat. No. 3,326,967, R. Appel and H. Gerber,
Chem.Ber. 91, 1200-1203 (1958), N. Onodera, Kogyo
Kagaku Zasshi 65, 790-793 (1962), U.S. Pat. No.
3,420,867.
It has been unexpectedly found that the use of the
adhesive compositions containing the sulfamide deriva-
tives of general formula I according to the present in-
vention exhibit improved stability during storage. Due
to the advantageous action of the stabilizers presently
described, it is only necessary to add small quantities of
0.0001 to 0.1% by weight and preferably 0.001 to 0.05%
by weight (based on the total weight of the adhesive
compositions) to the a-cyanoacrylate adhesives to ob-
tain excellent storage properties. In addition, it has been
found that the curing rate of a-cyanoacrylate adhesive
compositions is not impaired by adding the stabilizers
according to the present invention.
As mentioned before conventional additives can be
added to the adhesive compositions according to the
invention. Thus, common inhibitors for the radical pol-
ymerization can be added to the stabilized oL-cyanoacry-
late-based adhesives. Such inhibitors are for example
quinone, hydroquinone, p-methoxyphenol, pyrogallol
etc. These inhibitors can be added in a concentration of
0.0001 to 1% by weight based on the total weight of the
adhesive composition.
The properties of the adhesive compositions accord-
ing to the invention can be further improved by adding
phenolic stabilizers (anti-oxidants). The phenolic anti-
oxidants can be added in the same amounts as the sulfa-
mide derivatives, but depending from the storage condi-
tions and the proposed use of the adhesive composition
also smaller or greater amounts of phenolic stabilizers
can be used. Accordingly 0.0001 to 1% by weight of
phenolic stabilizer based on the total weight of the ad-
hesive composition are mostly sufficient.
Phenols according to the following general formula
on 111
R8 R4
R7 R5
R6
are suitable as co-stabilizers; in the formula the radicals
R4,R5,R7 and R3 in each case stand for hydrogen or an
4,414,347
5
alkyl group with 1 to 4 carbon atoms and the radical R5
either has the same meaning or is a hydroxy or methoxy
group.
Preference is given to sterically hindered phenols,
2,5-di-tert.-butyl hydroquinone having proved particu-
larly advantageous.
As mentioned before the adhesive co_mpositions ac-
cording to the invention may contain conventional-
additives. Thus, for example when joining porous mate-
rials it is desired to increase the viscosity by adding a
thickener to avoid that the adhesive penetrates the
pores of the surfaces to be adhered to one another.
Furthermore, the setting times of the adhesive accord-
ing to the invention especially on porous/acidic sur-
faces can be reduced by polymerization catalysts like
podands as disclosed in German published applications
DE-OS No. 3,025,127 and DE-OS No. 3,109,220 which
are herein incorporated by reference. Such modifica-
tions are sufficiently described in the literature.
The following examples are given for illustrative
purposes only and are not meant to be a limitation on
the subject invention except as defined by the claims
appended hereto. All parts and percentages are by
weight unless otherwise indicated.
EXAMPLE 1
Preparation of N,N’-diacetyl-sulfamide
12 g of anhydrous acetic acid are dissolved in 150 ml
of absolute benzene and 14.8 g of sulfuryl diisocyanate
(prepared in accordance with German Pat. No. 940,351)
are added dropwise within 20 minutes at ambient tem-
perature, accompanied by stirring. The temperature
rises from 21° to 39° C. and C02 evolution as well as a
precipitation of a colorless precipitate occur. The reac-
tion mixture is heated by means of an oil bath to 60° C.
accompanied by stirring until the evolution of the gas is
at an end, which takes 2 hours. The reaction mixture is
cooled, the precipitate removed by suction filtering and
dried at 60° C. 17.8 g of colorless crystals are obtained
(98.8% of theory), ’m.p. 167°—l69° C. (decomp.) which
can be recrystallized from isopropanol and acetic acid.
The IR-spectrum shows bands at 3220 cm—‘ (NH-
stretching vibration), 1710 cm-1 (C:0-stretching vi-
bration) and 1490/1170 cm‘ (asymmetric and symmetric
S02-stretching vibration).
The 1H—NMR spectrum (DMSO-d6) has the follow-
ing absorptions: 8: 1.96 ppm, s, 6 H for the two CH3
groups, 8: 12.10 ppm, s wide, 2 H, exchangeable with
D20, for the two NH groups.
Elementary analysis: Calculated for C4HgN2O4S: C
26.66%; H 4,48%; N 15.55%. Found: C 26.64%; H
4.35%; N 15.65%.
The same process is used for the preparation of the
following N,N’-diacyl sulfamidesz
R Yield m.p.
H 91% 147-148" C.
ethyl 96% 152-153” C.
n-propyl 89% 148-151“ C.
cyclohexyl . 88% 179-182" C.
benzyl 84% l74~l75° C.
10
15
20
25
30
35
40
45
50
55
60
65
6
EXAMPLE 2
Reaction of sulfuryl diisocyanate with acetic acid in
tetrahydrofuran
36 g of anhydrous acetic acid-are placed in 200 ml of
absolute tetrahydrofuran and 44,4 g of sulfuryl diisocya-
nate are added dropwise within 20 minutes accompa-
nied by stirring. The temperature rises up to the reflux
temperature of thetetrahydrofuran. This is accompa-
nied by C02 evolution, which ends after 2 hours. The
tetrahydrofuran is evaporated in vacuo and the residue
in the form of a slightly cloudy, viscous, yellow oil is
diluted with absolute benzene. A small amount of yel-
low flakes forms and settles at the bottom. The clear
benzene solution is evaporated in vacuo. 100.1 g of a
yellow, viscous oil are left behind which does not crys-
tallize even after prolonged standing. A preparation
prepared in this way has all the properties of N,N’-dia-
cetyl sulfamide with regard to the stabilizing effect in
ct-cyanoacrylate-based adhesives. However, it has the
advantage of excellent solubility therein.
The trifluoromethyl derivative is prepared in the
same way.
EXAMPLE 3
Preparation of N,N’-bis-(benzyloxycarbonyl)-sulfamide
10 g of anhydrous benzyl alcohol are placed in 150 ml
of absolute benzene and 7.4 g of sulfuryl diisocyanate
are added dropwise accompanied by stirring. The exo-
thermic reaction which takes place leads to a rise in the
temperature to 45° C. and shortly after the start a pre-
cipitate is formed. Following the temperature drop,
stirring is continued for 1 hour at ambient temperature.
The resulting precipitate is suction filtered and dried at
60° C. giving a yield of 18.06 g of colorless crystals
(99.2% of theory), m.p. 139°—141° C., which can be
recrystallized from ethanol.
IR spectrum: 3290/3210 cm"1 (NH-stretching vibra-
tion), 1755 cm*1 (C20 stretching vibration),
1495/1150 cm'1 (asymmetric and symmetric S02
stretching vibration) and 1225 cm—l (C-0 stretching
vibration).
1H-NMR spectrum (acetone-d5): 8: 5.18 ppm, 5, 4 H
for the two —0CH2 groups, 8=7.35 ppm, s, 10 H for
the two phenyl radicals, 8=l0.7 ppm, s, wide, 2 H,
exchangeable with D20, for the two NH groups.
Elementary analysis: Calculated for C15H15N206S: C
52.74%; H 4.43%; N 7.69%. Found: C 52.40%; H
4.34%; N 7.74%.
The same process is used for the preparation in a
quantitative yield of the following addition products:
R m.p. (found)
—OCH3 163-165“ C.
-OC2l-I5 168-169” C.
—OC3H7(n) 137° C.
—OC4H9(n) 78—79" C.
-OC(CH3)3 152-153” C.
-0C6}-I5 153—l54° C.
EXAMPLE 4
Several stabilizers according to the invention as
stated in table 1 were added to freshly distilled mono-
meric ethyl-2-cyanoacrylate containing 0.01% by
weight hydroquinone and 20 ppm S02. The obtained
adhesives were thickened with polymethy1methacry1-
4,414,347
7
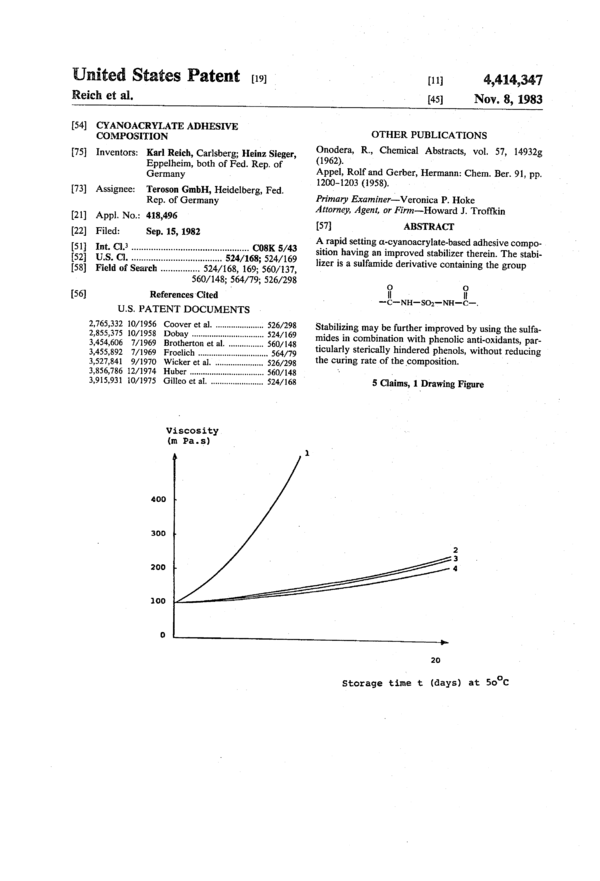
ate and tested in an accelerated ageing test at 50° C. The
change of viscosity as a measure for the stability is plot-
ted in FIG. 1.
After 20 days of accelerated ageing the stabilized 5
on-cyanoacrylate-based adhesives according to the in-
vention only exhibited a minor increase in viscosity and
save short setting times on various substrates before and
also after accelerated ageing.
10
In the same manner methyl-, C3—C2o-alkyl—, cycloal-
kyl-, alkoxyalkyl- and phenyl-2-cyanoacrylate as well as
mixtures of these esters were tested. The results ob-
tained were basically the same. 15
TABLE 1
Stabilizer Thickener
Sulfamide concentration PMMA
derivative (ppm) (% by weight) 20
O 0
II II
R—C—NH—SO2—NH-C-R
R = 25
I. no stabilizer — 5
2. methyl 100 s
3. benzyloxy 100 5
4. trifluoromethyl 100 5 30
——.
While the invention has been described in connection
with certain preferred embodiments, it is not intended
to limit the invention to the particular form set forth,
but, on the contrary, it is intended to cover such altema-
tives, modifications and equivalents as defined by the
appendend claims. '
What we claim is:
1. An a-cyanoacrylate-based adhesive compositions
containing a sulfamide compound of the general for-
mula
35
45
50
55
65
0 0
II II
R1—c—NH—so2—NH—c—R'
in which the groups R1 are each a radical separately
selected from the group consisting of hydrogen, linear
or branched alkyl with 1 to 18 carbon atoms, cycloalkyl
with 3 to 8 carbon atoms, phenyl methyl, trifluoro- or
trichloromethyl or alkoxy -—OR2, R2 being selected
from the group consisting of linear or branched alkyl
with 1 to 18 carbon atoms, cycloakyl with 3 to 8 carbon
atoms, phenyl, di- or triphenylmethyl, phenylalkyl with
l to 4 carbon atoms in the alkyl group, or alkylphenyl
with 1 to 4 carbon atoms in the alkyl radical.
2. The adhesive composition of claim 1 in which the
sulfamide derivative is the reaction product of sulfuryl
diisocyanate with a carboxylic acid RICOOH (in which
R1 is defined as above but may not be an alkoxy radical)
in tetrahydrofuran.
3. The adhesive composition of claim 1 wherein said
composition contains 0.0001 to 0.01% by weight of the
sulfamide derivative.
4. The adhesive composition according to claim 1, 2
or 3, which additionally contains a phenolic anti-oxi-
dant.
5. The adhesive composition of claim 4 wherein the
.phenolic anti-oxidant is a phenol of the general formula
OH
R3 R4
R7 R5
R6
in which R4, R5, R7 and R3 are each a radical selected
from hydrogen and lower alkyl with l to 4 carbon
atoms, and in which the group R5 is a radical selected
from the group consisting of hydrogen, lower alkyl
with l to 4 carbon atoms, hydroxy or methoxy.
# * i If *
Coments go here:
- Log in to post comments