Carbonates carrying cyclic carbonate groups
Carbonates carrying cyclic carbonate groups
US4423235
Year:
Abstract:
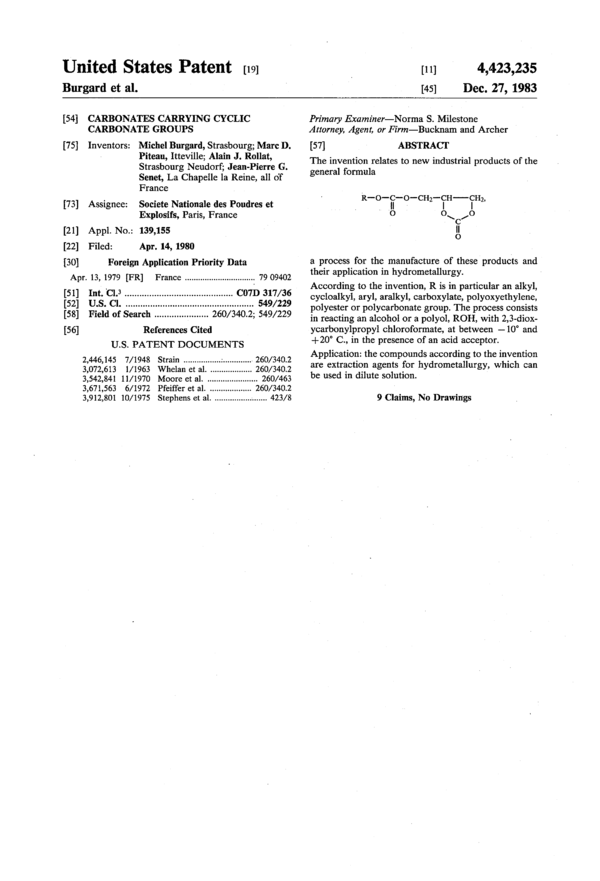
The invention relates to new industrial products of the general formula ##STR1## a process for the manufacture of these products and their application in hydrometallurgy.
Type of document:
Language:
United States Patent [19]
Burgard et al.
[54] CARBONATES CARRYING CYCLIC
CARBONATE GROUPS
Michel Burgard, Strasbourg; Marc D.
Piteau, Itteville; Alain J. Rollat,
Strasbourg Neudorf; Jean-Pierre G.
Senet, La Chapelle la Reine, all of
France
Societe Nationale des Poudres et
Explosifs, Paris, France
[21] Appl. No.: 139,155
[75] Inventors:
[73] Assignee:
[22] Filed: Apr. 14, 1980
[30] Foreign Application Priority Data
Apr. 13, 1979 [FR] France .............................. .. 79 09402
[51] Int. Cl.3 .......................................... .. C07D 317/36
[52] U.S. Cl. .................................... .. 549/229
[58] Field of Search .................... .. 260/340.2; 549/229
[56] References Cited
U.S. PATENT DOCUMENTS
2,446,145 7/1948 Strain ................ .; ........... .. 260/340.2
3,072,613 1/1963 Whelan et al. . .. 260/ 340.2
3,542,841 11/1970 Moore et al. 260/463
3,671,563 6/1972 Pfeiffer et al. .. .. 260/ 340.2
3,912,801 10/1975 Stephens et al. ...................... .. 423/8
[11] 4,423,235
[45] Dec. 27, 1983
Primary Examiner—Norma S. Milestone
Attorney, Agent, or Firm—Bucknam and Archer
[57] ABSTRACT
The invention relates to new industrial products of the
general formula
R—o—fi:—o—cH2—$H——$H2,
‘o o o
a process for the manufacture of these products and
their application in hydrometallurgy.
According to the invention, R is in particular an alkyl,
cycloalkyl, aryl, aralkyl, carboxylate, polyoxyethylene,
polyester or polycarbonate group. The process consists
in reacting an alcohol or a polyol, ROH, with 2,3-diox-
ycarbonylpropyl chloroformate, at between — 10° and
+20° C., in the presence of an acid acceptor.
Application: the compounds according to the invention
are extraction agents for hydrometallurgy, which can
be used in dilute solution.
9 Claims, No Drawings
4,423,235
1
CARBONATES CARRYING CY CLIC CARBONATE
GROUPS
The present invention relates to compounds carrying
cyclic carbonate groups, to the processes for the manu-
facture of these compounds and to their application in-
hydrometallurgy.
It is known from U.S. Pat. No. 2,446,145 to prepare
2,3-dioxycarbonylpropyl chloroforniate by reacting
phosgene with glycerol. It is also known, from the same
document, to obtain the carbamate and corresponding
urethanes by reacting this chloroformate with ammonia
or amines.
Furthermore, the value of certain organic substances
having the ability wholly or partially to extract metal
ions present in aqueous solutions is known. Such ore-
leaching solutions, from solutions resulting from attack
on recycled scrap metals or, alternatively, from indus-
trial effluents which are harmful to the environment.
Basically, these organic substances, which are re-
ferred to as extraction agents, must be capable not only
of more or less selectively extracting metal ions from
aqueous solutions, but also of returning them to a new,
pure and more concentrated aqueous solution which is
then treated in a manner which is in itself known.
However, in order to enable all the advantages pecu-
liar to hydrometallurgical techniques to be derived
from the extraction agents (versatility, production of
metals of high purity, automation, continuous operation
and, in particular, utilisation of the ores or waste), the
said extraction agents must also possess a number of
other properties, namely low solubility in the aqueous
phases (a cause of loss), low volatility (also a cause of
loss), stability (a further cause of loss), good solubility in
inexpensive organic media, good reversibility, high
extraction rate, selectivity (in the case of complex solu-
tions), high extraction capacity, high coefficient of ex-
traction, good resistance to recycling, low or zero tox-
icity and low or zero corrosive character.
In an article appearing in the review HYDRO-
METALLURGY in l976, Volume 1, pages 207-240,
and entitled “Solvent Extraction of Non-ferrous met-
als”, D. S. FLETT and D. R. SPINK focus on the main
extraction agents known and give their spectrum of
activity and their conditions of use in each case. A
distinction is thus made between solvating, acid, chelat-
ing and ionic extraction agents. The solvating extrac-
tion agents include phosphates, such as tributyl phos-
phate (TBP), ketones, such as methyl isobutyl ketone
(or MIBK) or isophorone (U .S. Pat. No. 4,008,308), and
ethers, such as glycol dibutyl ether (BUTEX). The acid
extraction agents’ include naphthenic and versatic acids
and, in particular, di-(2—ethylhexyl)—phosphoric acid (or
DZEHPA) (for example U.S. Pat. No. 3,989,607 and
French Pat. Nos. 2,342,346 and 2,367,832). The chelat-
ing extraction agents essentially include a-hydroxyox-
imes (commercially known under the name LIX),
which are described in U.S. Pat. Nos. 3,224,873 and
3,449,066. Finally, the ionic extraction agents mainly
include amines and quaternary ammonium salts, such as
those described in French Pat. No. 1,266,363.
The invention itself relates to new chemical com-
pounds which, in their application as extraction agents,
do not resemble any known type and thereby have a
particular spectrum of application, which is suitable for
providing solutions to general or specific problems in
the field of extraction.
10
15
20
25
30
35
40
45
50
55
60
65
2
The carbonates carrying cyclic carbonate groups,
according to the invention, have the general formula:
R-0--C--O—CH;_-CH——CH2 (A)
II I I
0
in which R is a linear or branched alkyl group which
contains from 1 to 20 carbon atoms and is optionally
substituted by one to three groups
—OCOO-CI-12"‘ CH— CH2,
I I
o—co——o
an alicyclic group which contains 4 to 20 carbon atoms
and is optionally substituted by one to three groups
——0—c—o—cH2-—cH—cH2,
II I I
0
an aralkyl group which contains from 7 to 20 carbon
atoms and is optionally substituted by one to three
groups
—o—c—o—cH2—cH——'-CH2,
II I I
o
a carboxylate group which contains from 2 to 20 carbon
atoms and is optionallysubstituted by a group
—O—C-0-CH2—CI-I-—CH2,
II — I I
o
a polyoxyethylene of the formula
R'—O—CH7_—CI-12),,
in which n is between 1 and 40 and in which R’ is a
hydrocarbon group containing from 1 to 10 carbon
atoms and optionally carries one or two chains
~—O—CH2——CH2),,', in which n’ is between 1 and 40,
which chains are terminated by a group
—o—c—o—cH;—cH—cH2,
II I I
o
a polyoxypropylene of the formula
R’-(-O-'CH2—?H-)5,
CH3
4,423,235
3
in which p is between 1 and 40 and in which R’ is a
hydrocarbon group containing from 1 to 10 carbon
atoms and optionally carries one or two chains
'—('0—CH2-C_—_O (chloroformate) at 1,780 30
cm*1 and v>%O (cyclic carbonate) at 1,800 cm—1
EXAMPLE 2
Preparation of 2,3-Dioxycarbonylpropyl n-Octyl
Carbonate
346 g (1.9 mols) of the chloroformate prepared above,
247 g (1.9 mols) of n-octanol and 600 ml of methylene
20
25
35
8
NMR spectrum agrees with the formula.
It should be noted that more volatile or less volatile 1
alkyl carbonates can be synthesised with the same ease;
under the same conditions and with a comparable suc-
cess to that which has just been reported.
EXAMPLE 3
Synthesis of trimethylolpropane tri-(-2,3-dioxycar-
bonylpropyl carbonate). .
93.8 g (0.7 mol) of trimethylolpropane, 400 cm3 of
acetone and 183 g (2.31 mols) of pyridine are introduced
into a 2 liter reactor. The temperature is kept at about 0°
C. and a solution of 417 g (2.31 mols) of 2,3-dioxycar-
bonylpropyl chloroformate in 400 cm3 of acetone is
added. After stirring for two hours at ambient tempera-
ture, the reaction mixture is filtered, the filtrate is con-
centrated under reduced pressure, the residue is taken
up in 1.5 liters of methylene chloride and the solution is
washed with acidified water and then with pure water.
The organic solution is dried, filtered and concentrated
under reduced pressure. 347 g (yield 88%) of trimethyl-
olpropane tri-(2,3-dioxycarbonylpropyl carbonate) are
thus obtained.
Melting point: 120° C. Residual OH level: 0%
Infra-red spectrum:
v E (linear carbonate): 1,750 cm'‘1
0
v OIIIJ (cyclic carbonate): 1,800 cm‘1
0
This spectrum is in total agreement with theory.
EXAMPLE 4
Synthesis of tetraethylene glycol di-(2,3-dioxycar-
bonylpropyl carbonate).
CH2-— CH— CH2'—O-C’-O-('CH2'— CH2--O')2;-C-0-' CH2-' CH——CH2
! I
o
chloride are introduced into a reactor equipped with a
reflux condenser, a thermometer, a stirrer and a drop-
ping funnel.
152 g (2.2 mols) of pyridine (that is to say a molar
excess of 15%) are run in over a period of about 1 hour,
whilst keeping the temperature between —5° and 5° C.
The mixture is stirred for 1 hour at 0° C. and is then
left to return to ambient temperature in the course of
about 2 hours.
The pyridine hydrochloride is removed by filtration 55
and the organic phase is washed with twice 500 ml of
water. ‘ '
After drying over magnesium sulphate and removing
the solvent by evaporation under reduced pressure, the
product is topped at 100° C. under a pressure of 1 mm
Hg for 30 minutes.
460 g of a colourless oil, which crystallises at ambient
temperature, are thus obtained. The yield is 88% rela-
tive to the chloroformate.
Analyses:
Infra-red spectrum: absorption bands
vC-:0 (linear carbonate) at 1,740 cm—1 and
vC=O (cyclic carbonate) at 1,800 cm-1
50
60
65
O\ /O
C
II
0
II II
0 0
2.888 kg (16 mols) of 2,3-dioxycarbonylpropyl chlo-
roformate and 5 liters of anhydrous methylene chloride
are introduced into a 20 liter glass reactor.
The temperature is kept between -4“ and +2° C.
and a mixture of 1.552 kg (8 mols) of tetraethylene
glycol, 1.430 kg (18.1 mols) of pyridine (that is to say an
excess of 13% relative to stoichiometry) and 1 liter of
anhydrous methylene chloride is run in slowly, whilst
stirring.
After the introduction, which takes 1 hour 45 min-
utes, the reaction mixture is heated to between +15°
and + 18° C. and this temperature is maintained for one
hour.
7 liters of salt water are added and the organic phase
is separated off. The latter is washed three times with a
mixture of 7 liters of salt water and 500 ml of concen-
trated hydrochloric acid, twice with 7 liters of salt
water and once with 7 liters of demineralised water.
The organic phase is dried over anhydrous sodium
sulphate and the solvent is removed by evaporation
under reduced pressure at 50° C. '
4,423,235
9 10
3.225 kg (yield 83.6%) of a very viscous product are
thus collected, the main characteristics of this product EXAMPLE 6
being as follows: Synthesis of poly—[(diethy1ene glycol) carbonate]
water content: 0.040% di-(2,3-dioxycarbonylpropyl carbonate)
cH2—CH-cH;o—c—o—(-CH2-—CH2-o—cH1—cH2—o-c—oa-,;—cH2——cH———cH2
I I II II I I
o 0 o o 0 o
\ C / \C./
‘ II II
0 O
m = 10
content of residual OH groups §10—2 eq/kg The procedure used is strictly identical to the above
Tetraethylene glycol di—(2,3-dioxycarbonylpropyl procedure (Example 5), using 410 g (0.33 mol) of poly-
carbonate) 15 (diethylene glycol carbonate), 58 g (0.73 mol) of pyri-
Cill-lz—-'CH"'CH2— O—C—O'f'CH2— CI-12- O‘)trC-" 0- CH2—(|3HjC|IH2
II II
o\ /o 0 o o\ /o
C
II ' II
0
Viscous liquid, slightly amber—coloured, possessing a dine (that is to say an excess of 10% relative to stoichi-
slight odour: 25 ometry), 600 ml of anhydrous methylene chloride and
Melting point: 14° C. (transition point) 132 g (0.73 mol) of 2,3-dioxycarbonylpropyl chlorofor-
Vapour pressure mate.
at 20° C.: 5 mm Hg . 435 g of product are thus collected, the infra-red
at 100° C.: 22 mm Hg spectrum of this product agrees with the formula given
Density d2525: 1.41 30 above and its number—average molecular weight is
Viscosity at 25° C.: 60,000 cP about 1,500, its content of OH groups is less than or
Refractive index at 25° C., nD25: 1.4750 equal to 0.02 eq/kg and the total chlorine level is 0.06%.
Solubility: insoluble in water, good solubility in or-
ganic solvents (60 g in 100 g of CH2C12 at 20° c.) EXAMPLE 7
Synthesis of 2,3-dioxycarbonylpropyl (ethyl
35
EXAMPLE 5 methacrylate) carbonate
Synthesis of poly-(ethy1eneglycol)di-(2,3-dioxycar-
bonylpropyl carbonate) 11.4 g (0.1 mol) of 2-hydroxyethyl methacrylate, 9 g
(0.11 mol) of pyridine and 50 ml of 2,3-dioxycarbonyyl-
Coments go here:
- Log in to post comments