Use of t-butyldimethylsilyl cyanoacetate for preparation of α-cyano ketones
Use of t-butyldimethylsilyl cyanoacetate for preparation of α-cyano ketones
Journal:
Year:
Abstract:
The synthesis of t-butyldimethylsilyl cyanoacetate and the reactions of its anion with acyl donors are described. The reagent was found to be the method of choice for the syntheses of α-cyano ketone substrate analogues for carboxypeptidase A. These compounds have been shown to be potent mechanism-based inactivators for the enzyme.
DOI:
10.1002/bip.360290115
Type of document:
Language:
Use of t-Butyldimethylsilyl Cyanoacetate for
Preparation of a-Cyano Ketones
SOUMITRA S. GHOSH
T h e Salk Institute Biotechnology/lndustrial Associates, Inc., P.O. Box 85200,San Diego, California 92138-9216
SYNOPSIS
The synthesis of t-butyldimethylsilyl cyanoacetate and the reactions of its anion with
acyl donors are described. The reagent was found to be the method of choice for the
syntheses of a-cyano ketone substrate analogues for carboxypeptidase A. These compounds
have been shown to be potent mechanism-based inactivators for the enzyme.
INTRO D UCTlON
A major objective of our research has been the
design of mechanism-based inactivators for the enzyme, carboxypeptidase A (CPA). The key element
of the strategy was the observation by Kaiser and
his co-workers that CPA can catalyze the stereospecific abstraction of protons from activated
methylene groups of ketonic substrate analogues.’-4
This enzyme-assisted enol formation was exploited
to carry out a,P elimination reactions to produce
Michael acceptors, which were chemically inert,
however, in the active ~ i t e . More recently, we
~.~
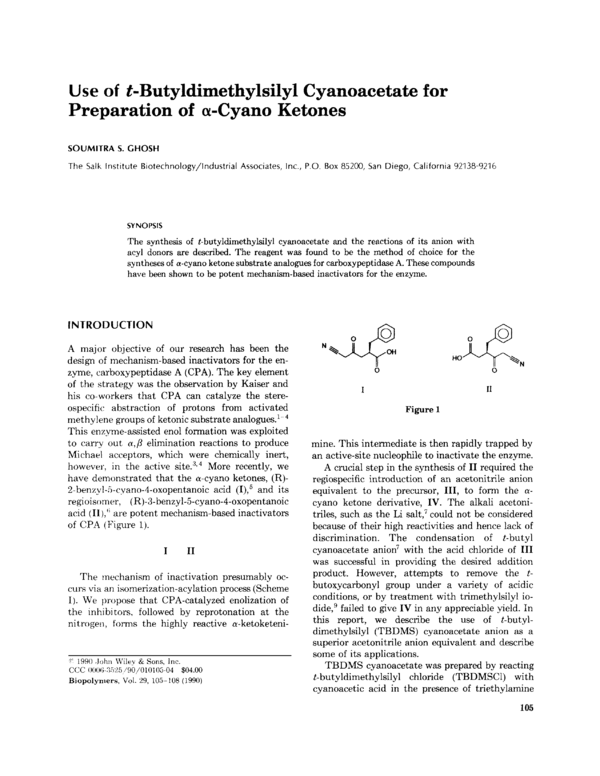
have demonstrated that the a-cyano ketones, (R)2-benzyl-5-cyano-4-oxopentanoic (I),5and its
acid
regioisomer, (R)-3-benzyl-5-cyano-4-oxopentanoic
acid (I1 are potent mechanism-based inactivators
of CPA (Figure 1).
),‘I
I
I1
The mechanism of inactivation presumably occurs via an isomerization-acylation process (Scheme
I). We propose that CPA-catalyzed enolization of
the irihibi tors, followed by reprotonation at the
nitrogen, toms the highly reactive a-ketoketeni-
1990 ,John Wiley & Sons, Inc.
O(IOfi 3325 /90/OlOlO5-04
$04.00
Biopolyniers, Vol. 29, 105-108 (1990)
‘c
CCC
I
I1
Figure 1
mine. This intermediate is then rapidly trapped by
an active-site nucleophile to inactivate the enzyme.
A crucial step in the synthesis of I1 required the
regiospecific introduction of an acetonitrile anion
equivalent to the precursor, 111, to form the acyano ketone derivative, IV. The alkali acetonitriles, such as the Li salt,7could not be considered
because of their high reactivities and hence lack of
discrimination. The condensation of t-butyl
cyanoacetate anion7 with the acid chloride of 111
was successful in providing the desired addition
product. However, attempts to remove the tbutoxycarbonyl group under a variety of acidic
conditions, or by treatment with trimethylsilyl iodide,9 failed to give I V in any appreciable yield. In
this report, we describe the use of t-butyldimethylsilyl (TBDMS) cyanoacetate anion as a
superior acetonitrile anion equivalent and describe
some of its applications.
TBDMS cyanoacetate was prepared by reacting
t-butyldimethylsilyl chloride (TBDMSC1) with
cyanoacetic acid in the presence of triethylamine
105
106
GHOSH
\I/
Zn++
\I/
zn++
Scheme I
Scheme I1 (a) (COCI),, DMF (b) [NCCHCO,TBDMSI-Na' and (c) H'.
NCCH,CO,H
TBDlllS (21
NCCH,CO,TBDMS
Figure 2
(Figure Z).'O The silyl ester was isolated in 90%
yield by filtration and then concentration of the
filtrate under vacuo. Reaction of the sodium salt of
the reagent with freshly prepared acid chloride of
111, followed by acid quench, directly provides the
a-cyano ketone derivative (Scheme 11; 48% overall
yield from 111). Similarly, the a-cyan0 ketone
derivative of the half ester of benzylmalonic acid
was obtained in 38% overall yield.
The reactivity of the TBDMS cyanoacetate anion toward anhydrides is modulated by the nature
of its countercation. Thus, while its Na salt did not
react a t all with (R)-benzylsuccinic anhydride, the
Li derivative added facilely to give a 1:1 mixture
of I and I1 in 87%isolated yield after silica gel flash
chromatography (Figure 3). The two regioisomers
are well resolved by C-8 semi-preparative reversephase high performance liquid chromatography
(HPLC). The efficacy of the reagent was borne out
in the subsequent syntheses of 14C-labeledI and 11.
14
C-labeled TBDMS cyanoacetate was prepared in
two steps by reacting chloracetic acid with 14CNaCN," followed by esterification with TBDMSCI. Reaction of its Li salt with (R)-benzylsuccinic
anhydride and then purification by reverse-phase
chromatography afforded the desired radiolabeled
compounds. This sequence of reactions is far more
economical than the use of the very expensive
14
C-labeled acetonitrile.
THDMS cyanoacetate was designed to allow a
facile one-step introduction of a CH,CN group by
taking advantage of the known acid lability of
TBDMS esters. In its reactions with acid chlorides
and anhydrides, the silyl esters of the initially
formed products rapidly hydrolyze under the acidic
work-up conditions and then decarboxylate to give
rise to a-cyano ketones. The ease of its synthesis
and application recommends its use as an attractive alternative to other synthetic methods for the
preparation of cyanomethylated compounds.
EXPERIMENTAL
Infrared spectra were obtained on a Perkin Elmer
1420 spectrometer or a Mattson Cygnus 25 Fourier
transform (FT) ir instrument. Optical rotations
were taken on a Perkin Elmer 241 polarimeter.
Nuclear magnetic resonance spectra were recorded
on a Nicolet 360 MHz FT-nmr using tetramethylsilane as an internal standard. All mass spectra were
USE OF TBDMS CYANOACETATE
obtained a t the Rockefeller University Biotechnology Mass Spectrometric Research Resource.
(TB DMS) Cyanoacetat e
To a solution of cyanoacetic acid (1.7 g, 20 mmoles)
and TBDMS chloride (3.14 g, 20 mmoles) in 22 mL
of anhydrous ethyl acetate at 0°C and under N,
was added 2.71 mL of triethylamine, resulting in
the immediate precipitation of triethylamine hydrochloride. The reaction mixture was stirred a t
0°C for 30 min and then allowed to warm to an
ambient temperature. The suspension was filtered,
and the salt precipitate was washed with ethyl
acetate (2 x 20 mL). The filtrates were combined
and concentrated to afford 3.55 g (90%) of the
TBDMS ester as a clear oil. 'H-nmr (CDC1,): 6
3.47 (s, 2H), 0.96 (s, 9H), 0.32 (s, 6H); ir (thin film)
3455, 2934, 2862, 2266, 1731, 1471 cm-'.
(R)-2-Benzyl-3-Carbomethoxy-Propionyl Chloride
To 0.33 g (1.5 mmoles) of (R)-2-benzyl-3-carbomethoxy-propionic acid in 19 mL of anhydrous
benzene were added 0.019 mL of dimethylformamide (DMF) and 0.163 mL (1.88 mmoles) of
oxalyl chloride, resulting in rapid gas evolution.
After stirring for 30 min a t 23"C, the solution was
concentrated, taken up in 20 mL tetrahydrofurane
(THF), and evaporated once again to ensure removal of unreacted oxalyl chloride. Traces of solvent were removed under high vacuum, and the
acid chloride was used immediately for the next
step without further purification.
(R)-Methyl 3-Benzyl-4-0~0-5-Cyano-Pentanoate
(IV)
To 0.072 g (3 mmoles) of sodium hydride in 30 mL
of anhydrous THF under N, was added a solution
of 0.591 g (3 m o l e s ) of TBDMS cyanoacetate in 5
mL THF over 5 min, and the reaction was allowed
to proceed for 15 min. The reaction mixture was
then cooled to - 78"C, and a solution of the (R)-2benzyl-3-carbomethoxy-propionyl
chloride (max 1.5
mmoles) in 7 mL of THF was added dropwise over
a period of 15 min. After stirring a t -78°C for 30
minutes, the mixture was warmed to 23°C over 30
minutes and then quenched with 27 ml of 0.06 N
HCl. The solution was extracted with ethyl acetate
(3 x 40 mL), and the organic phase was washed
with brine, dried, and concentrated. Two drops of
triethylamine were added to the residue, and the
mixture was purified by flash chromatography.
107
Elution with 30% ethyl acetate in hexane containing 0.5% triethylamine separated a minor contaminant, which was followed by elution with ethyl
acetate to afford 172 mg (48% from the acid) of the
product as an oil. 'H-nmr (CDCI,): 6 7.37-7.14 (m,
5H), 3.64 (s, 3H), 3.54 (d, J = 19.7 Hz, lH), 3.31
(m, lH), 3.05 (d, J = 19.7 Hz, lH), 2.89 (m, lH),
2.71 (dd, J = 13.3, 7.1 Hz, lH), 2.52 (dd, J = 17.6,
3.5 Hz, 1H); ir (thin film) 3028, 2953, 2261, 1730,
1438 cm-'. HRMS (CI) calculated for C,,H160,N
(M + 1) 246.1130, found 246.1126. [ a ] : + 75.2 (c
0.7, ethyl acetate).
Syntheses of I and I1
To 0.414 mL of a 2.41M solution of butyl lithium
(1 mmole) under argon were added 1 mL of anhydrous THF and then 0.14 mL (1 mmole) of diisopropylamine, and the mixture was stirred for 15
min a t 0°C. The solution was cooled to -78"C,
and a solution of 0.203 g of TBDMS cyanoacetate
in 2 mL of THF was added over 5 min. After
stirring for 30 min, a solution of 0.094 g (0.5 mmoles)
of (R)-benzylsuccinic anhydride in 3 mL of THF
was added over a period of 5 min. The mixture was
stirred a t -78°C for 1h and then a t 4°C for 3.5 h.
The reaction was quenched by the addition of 0.5
mL of 10% HC1, taken up in 40 mL of ether, and
washed with 15 mL of water. The aqueous layer
was extracted with 2 x 15 mL of ether, and then
the ethereal solutions were combined, washed with
20 mL of brine, dried over MgSO,, and concentrated under vacuo. The crude products were purified by flash chromatography using 50% ethyl acetate in hexane containing 0.5% acetic acid to afford 0.1 g of a 1:l mixture of I and I1 as an oil (87%
yield).
Spectral Data for I. 'H-nmr (CDCI,): S 7.34-7.16
(5H, m), 6 3.37 (2H, bs), 6 3.32-3.17 (2H, m), 6
2.83-2.76 (2H, dd, J = 9.3, 13.5 Hz), S 2.54--2.48
(lH, dd, J = 4.4, 17.4 Hz); ir (thin film) 3200 (br),
2916, 2264, 1731, 1713 cm-'. MS (CI) 232 (M + 1).
Spectral Data for 11. 'H-nmr (CDCl,): 6 7.37-7.14
(m, SH), 3.41 (br.d, J = 18.6 Hz, lH), 3.28 (m, lH),
3.01 (d, J = 19.7 Hz, lH), 2.89 (m, 2H), 2.73 (dd,
J = 13.4, 7.2 Hz, lH), 2.55 (dd, J = 17.9, 3.7 Hz,
1H); ir (thin film) 3500-3000 (br), 2263, 1730, 1393
cm-'. HRMS (CI) calculated for C,,H,,O,,N (M +
1) 232.0974, found 232.0970. [a]$ + 77.8 (c 0.7,
ethyl acetate).
10s
GHOSH
This work was supported by funds from SIBIA. I thank
Dr. Shahriar Mobashery for helpful discussions, and I
owe a debt of gratitude to Dr. Tom Kaiser, without
whom this work would not have been possible.
REFERENCES
1. Sugimoto, T. & Kaiser, E. T. (1978) J . Am. Chem.
SOC.
100,7750-7751.
2. Sugimoto, T. & Kaiser, E. T. (1979) J . Am. Chem.
SOC.101, 3946-3951.
3. Nashed, N. T. & Kaiser, E. T. (1981) J . Am. Chem.
SOC.103, 3611-3612.
4. Nashed, N. T. & Kaiser, E. T. (1986) J . Am. Chem.
SOC.108, 2710-2715.
5. Mobashery, S., Ghosh, S. S., Tamura, S. Y. & Kaiser,
E. T., Proc. Natl. Acad. Sci. U.S.A. (in press).
6. Ghosh, S. S., Spratt, T. E., Miller, W. T. & Kaiser,
E. T., submitted.
7. Kaiser, E. M. & Hauser, C. R. (1968) J . Org. Chem.
33, 3402-3404.
8. Lawsson, S.-O., Larsen, E. H. & Jacobsen, H. J.
(1965) Arkiu. Kemi. 23, 453-462.
9. Jung, M. E. & Lyster, M. A. (1977) J . Org. Chem. 99,
968-969.
10. Mobashery, S. &Johnston, M. (1985) J. Org. Chem.
50, 2200--2202.
11. Inglis, J. K. H. (1932) in Organic Syntheses collective
Vol. I, Gilman, H. & Blatt, A. H., Eds., Wiley, New
York, pp. 254-256
Received May 4,1989
Accepted June 9, 1989
Coments go here:
- Log in to post comments